Oct 3 2008
Carbon atoms are the building blocks for millions of organic molecules, yet this variety is built on the simple rule that carbon almost always shares a total of four chemical bonds with its neighbors. Now, an international team of chemists has mapped a highly unusual compound containing a carbon atom that hooks up to six atoms at once*.
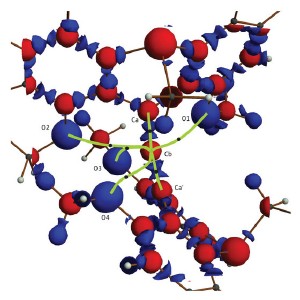 This map of the electron density in the molecule shows that the central carbon atom (Cb) is connected by six bonds (green).
This map of the electron density in the molecule shows that the central carbon atom (Cb) is connected by six bonds (green).
The chemical bonds in organic compounds are made from pairs of electrons, whose negative charge sticks atoms together. Atoms with more than four chemical bonds are called hypervalent, which is common in elements such as phosphorus and sulfur, but very rare in carbon.
Hypervalent carbon atoms are not just a curiosity, explains team-member Daisuke Hashizume of RIKEN’s Advanced Science Institute in Wako. “Carbon is the most important atom in chemistry,” he says. When carbon-based organic molecules react, the atomic rearrangement involved usually creates short-lived hypervalent intermediates. Studying more stable hypervalent compounds can help to explain why certain chemical reactions proceed in particular ways.
Scientists at Hiroshima and Waseda Universities first created a compound containing a central carbon atom connected to two flat anthracene groups that carried a total of four dangling oxygen atoms. A chemical reaction then added positive charges to the anthracene groups, drawing electrons from the oxygen atoms to form a bond with the central carbon atom, making it hypervalent.
Then Hashizume’s team—working with colleagues at the University of California, Riverside, and the Rigaku Corporation—analyzed exactly how electrons were distributed in the molecule, to confirm its bonding pattern.
The team used x-ray diffraction at RIKEN’s SPring-8 Center to map the positions of atoms and the electron density in the molecule, and confirmed that the oxygen atoms were close enough to the carbon to form genuine chemical bonds. They also found that electrons originally located around the oxygen atoms had shifted towards the central carbon atom. These bonding electron pairs are spread between three atoms, as opposed to the usual two, says Hashizume.
Although some hexavalent carbon compounds are already known, this is the first time that such a detailed analysis of how the electrons are distributed around the molecule has been done. “We have confirmed all six bonds on the carbon atom experimentally, not theoretically,” explains Hashizume (Fig. 1).
The results will help scientists to synthesize related compounds, says Hashizume, and to study reaction intermediates in more detail. “The next stage is to isolate and see the electronic structure of reaction intermediate species, to give us a deeper understanding of chemical reactions.”
- Yamaguchi, T., Yamamoto, Y., Kinoshita, D., Akiba, K., Zhang, Y., Reed, C.A., Hashizume, D. & Iwasaki, F. Synthesis and structure of a hexacoordinate carbon compound. Journal of the American Chemical Society 130, 6894–6895 (2008).