Although particle size and its measurement are intuitively familiar to particle technologists, the concept of zeta potential is less widely understood and applied. This is unfortunate since it is at least as fundamentally important as particle size in determining the behavior of particulate materials, especially those with sizes in the colloidal range below a micrometer. Zeta potential is related to the charge on the surface of the particle, and so influences a wide range of properties of colloidal materials, such as their stability, interaction with electrolytes, and suspension rheology.
What is Zeta Potential?
When a particle is immersed in a fluid, a range of processes causes the interface to become electrically charged. Some of the most commonly found charging mechanisms include adsorption of charged surfactants to the particle surface (for example in an emulsion stabilized by an ionic surfactant), loss of ions from the solid crystal lattice (silver halide particles used in photographic emulsions) and ionization of surface groups (carboxylate in polymer microspheres). These processes lead to the production of a surface charge density, expressed in coulombs per square meter, which is the fundamental measure of charge at the interface. The charge cannot be measured directly, but only via the electrical field it creates around the particle. Thus the surface charge is normally characterized in terms of a voltage at the particle surface, the surface potential, rather than a charge density, although one can usually be calculated from the other. The zeta potential occurs at a distance from the surface and this will be different to the surface potential. In the simplest approximation, the potential decays exponentially with distance from the surface of the particle (Fig. 1). As we will see, the rate of decay is dependent on the electrolyte content of the fluid.

Figure 1. Approximation of zeta potential as a function of distance from the particles’ surface.
How is Zeta Potential Measured?
So far, we have not defined zeta potential, and in order to do this we need to understand the basic method for its measurement, which is electrophoresis. To many, this method is familiar because of its use for the separation of macromolecules, and particle electrophoresis is a similar phenomenon. The particles in their suspending medium are placed in an electric field; if charged, they will drift in the field, positive particles drifting towards the negative electrode, and negative particles drifting towards the positive electrode. However, the particles do not drift on their own; they carry a thin layer of ions and solvent around them. The surface separating the stationary medium from the moving particle and its bound ions and solvent is called the surface of hydrodynamic shear, and the zeta potential is the potential at this surface.
Consequently zeta potential can be determined by measuring the drift velocity of the particle in an electrical field of known strength. Early instruments for this purpose (the Rank micro electrophoresis apparatus) used manual observation of the particles, a procedure that was fraught with error and also extremely slow. Fortunately, we now have a range of instruments that measure the velocity using the doppler shift of light scattered from the moving particles – the Malvern Panalytical Zetasizer series. Advance signal recovery techniques reliably measure the tiny doppler shift due to the particle movement (only a few tens of Hz in 1015 Hz) and automatically calculate the distribution of zeta potentials in the sample. Normally this value lies within the range +/- 100 mV for most systems immersed in aqueous media.

Figure 2. The Malvern Panalytical Zetasizer for measurement of zeta potential.
Zeta Potential and Electrolytes
One of the major uses of zeta potential is to study colloid-electrolyte interactions. Since most colloids, particularly those stabilised by ionic surfactants, are charged, it is not surprising that they interact with electrolytes in a complex manner. Ions of charge opposite to that of the surface (counterions) are attracted to it, while ions of like charge (co-ions) are repelled from it. Consequently the concentrations of ions near the surface are not the same as those in the bulk of the solution (i.e. at a long distance from the surface) as shown in Figure 3. The accumulation of counterions near the surface causes the particle charges to be screened, thus reducing the zeta potential. Ions can conveniently be divided into three classes depending on how they interact with the surface:
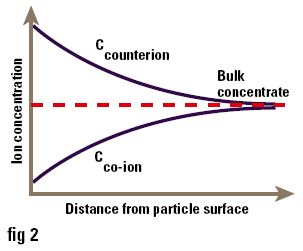
Figure 3. Concentration of ions near to the surface of a particle in solution.
Indifferent ions are those which are only attracted to the surface by virtue of their charge in a purely electrostatic manner, a process known as non-specific adsorption. If we measure the zeta potential of a colloid as a function of concentration of such an ion, we find that the screening effect of the ions gradually reduces the zeta potential (not the surface potential), and this asymptotes to zero at high electrolyte concentrations (Figure 4a).

Figure 4. Zeta potential as a function of electrolyte concentration for an indifferent electrolyte (a) and for a specifically adsorbed electrolyte (b).
Specifically adsorbed ions interact chemically with the surface, for example by complexation with groups on the surface. Consequently as their concentration is increased, they also screen the zeta potential, but the additional chemical (as distinct from electrostatic) binding on the surface causes sufficient adsorption of ions for the original particle charge to be neutralised and then reversed as the electrolyte concentration increases (Figure 4b). In such a system we see a point of zero charge or PZC at a well-defined electrolyte concentration, prior to charge reversal.
Potential Determining Ions
Potential-determining ions (PDI) are a special case of specifically adsorbed ions; this term is usually reserved for those involved in whatever process is responsible for the particle charge. For example, most polymer microspheres are charged because they have carboxylate groups on the surface; ionization of these groups leads to the charge, so H+ is a PDI on this surface. Similarly Ag+ and I- are PDI’s on silver iodide particles. The distinction between specifically adsorbed and potential determining ions is often vague, particularly in those systems in which the surface chemistry is not fully understood.
Zeta Potential and Flocculation
The major area of application of colloid-electrolyte phenomena is to understand stability and flocculation effects. The simplest model of these phenomena arises directly from Figure 4, and is known as the DLVO (Deryaguin- Landau-Verwey-Overbeek) theory. This simply states that the stability of the colloid is a balance between the attractive Van der Waals’ forces and the electrical repulsion due to the surface charge. If the zeta potential falls below a certain level, the colloid will aggregate due to the attractive forces. Conversely, a high zeta potential maintains a stable system. The point at which electrical and Van der Waals’ forces exactly balance can be identified with a specific electrolyte concentration, known as the critical flocculation concentration or CFC (Figure 5). Indifferent ions cause the zeta potential to continuously decline at high concentration, so we see a single CFC, and the colloid aggregates at all higher electrolyte concentrations. In contrast, specifically adsorbed ions cause charge reversal that may be sufficient to re-stabilise the colloid. In this case we will see an upper and lower CFC, with a region of instability between them.

Figure 5. The effect of electrolyte concentration on flocculation.
Studying Zeta Potential
The foregoing discussion shows us that the zeta potential measured in a particular system is dependent on the chemistry of the surface, and also how it interacts with its surrounding environment. This is a most important point; zeta potential must always be studied in well-defined environments (specifically pH and ionic strength) or the data is valueless. It is quite meaningless to talk about "the zeta potential of a surface" unless the conditions are specified. In order to illustrate the planning of a zeta potential study, it is useful to take a case study on a particular system. We have studied triglyceride fat emulsions for some years, and these studies provide a useful illustration of the power of zeta potential measurement in understanding colloid stability in complex systems.
Intravenous Fat Emulsions
Triglyceride emulsions are medical products; they are sub micron emulsions of vegetable oils in water, emulsified by phospholipids, which provide a high zeta potential, and a correspondingly long shelf life (2-3 years). The emulsions are used to feed patients intravenously who cannot be fed orally (e.g. due to gastrointestinal surgery). Such patients also need other nutrients, including amino acids, glucose and electrolytes. For some time it has been the common practice to mix all of these materials, in varying proportions, in a single liquid mixture (a total parenteral nutrition or TPN mixture) and infuse it into a patient, at a rate of about 3 litres a day. Naturally, in such a mixture, there is a wide scope for interaction between the components, and in many mixtures the fat emulsion becomes unstable, and coalesces or flocculates in a few days. In this condition it is unsuitable for infusion, and so the mixtures are normally made up just before administration, using sterile techniques. An understanding of the stability of the emulsion in these systems would be helpful in predicting which mixtures would be unstable, and even possible in producing stable mixtures with long shelf life.
Formulation Protocol
Early studies demonstrated that the emulsion itself, at a pH of 7 and low electrolyte concentration, had a zeta potential of –40 to –50 mV, which is sufficient to provide good stability and a shelf life of at least 2 years. This potential was markedly reduced by electrolytes, with monovalent cations being indifferent, while divalent cations adsorbed specifically with a PZC of 3 mM and a significant degree of charge reversal. These ions are all present in TPN mixtures, and this accounts for the instability of the emulsion in these systems.
Problems Correlating The stability of Emulsions with Zeta Potential
It should be possible to use the DLVO theory to correlate the stability of the emulsions in a particular mixture with its zeta potential; unfortunately there are a number of problems involved in making such a measurement. The mixtures contain a large phase fraction (1-5%) of the emulsion, and so are very turbid, and must be diluted before light scattering measurements can be performed. Early workers who did not understand the nature of zeta potential simply diluted the mixtures with distilled water. The resultant zeta potentials bore no resemblance to those of the emulsion in the original mixture since the dominant ions were reduced in concentration by some orders of magnitude! In order to obtain a relevant zeta potential it is necessary to maintain the continuous phase composition on dilution. There are two approaches to this problem; if the composition of the continuous phase is known, it can be prepared without any emulsion component and used as a diluent. A more common situation is that the continuous phase composition is uncertain; even if you knew what went into it, adsorption to the disperse phase may have depleted some components. In this case, the usual trick is to centrifuge the dispersion to get a clean sample of the continuous phase for dilution.
The second problem with this measurement is the extremely high ionic strength (0.2-0.4 M) which leads to high conductivity and consequently rapid sample heating and large cell voltage drops. The early Zetasizer 2 could not cope particularly well with this problem, but the current Zetasizer range has cell voltage pulsing that keeps the mean current down; and the reengineering of the electrophoresis cell has resulted in major improvements in electrical stability. It is now possible to use this instrument to routinely measure zeta potentials in these high conductivity mixtures, and the resulting values (± 1-5 mV) correlate well with the stability of the emulsion in the mixtures. Studies of this type are now allowing us to understand the behavior of emulsions in complex colloidal systems and provide real predictive power for formulation purposes.
Drug Targeting and Delivery Systems
Emulsions have also been used as drug delivery systems, and in many cases an understanding of the electrophoretic properties is crucial in formulation design. Although most drugs are water-soluble, an increasing number are surfaceactive or even hydrophobic, and such materials can provide significant problems for conventional formulation techniques. Consequently hydrophobic drug candidates are usually sent back to the chemistry department with a note to prepare a water-soluble analogue! In some cases this is not possible, for example some natural products or biotechnology materials, or where the mode of action is related to the lipophilicity, e.g. anaesthetics, hypnotics, and tranquilizers. In these cases emulsion delivery is increasingly used. Examples are ICI’s Diprivan, an intravenous anaesthetic, and Kabi’s Diazemuls, a sedative.
An example of the problems that can be encountered in this approach is shown in Figure 6, which is the zeta potential – pH curve for a drug-containing emulsion that is flocculated at pH 7. Data of this type allows a rational selection of formulation pH and emulsifier to maximize zeta potential and hence emulsion stability.

Figure 6. pH versus zeta potential data allowing optimization of emulsion stability.
Non-Aqueous Systems
A further example of the use of zeta potential in understanding suspension stability occurs in the suspensions of drugs in aerosol propellants used for delivery of drugs by inhalation, for example bronchodilators. The micronised drug is suspended in the aerosol propellant, so that when the aerosol is fired, particles of drug are sprayed out and can be inhaled. It is important to control the particle size by controlling the zeta potential, to guarantee a repeatable dose to the patient. The problem in this case is that it is extremely difficult to measure zeta potentials of particles suspended in nonaqueous media like CFC propellants, since the particle mobilities are very small. However, it can be done with appropriate design of the electrophoresis cell, and Malvern Panalytical make such a cell for their Zetasizer. Figure 7 shows the zeta potential of lactose (a model solid dispersion) in chloroform (a model non-aqueous medium) as a function of the concentration of lecithin, an ionic surfactant. The lecithin clearly causes major changes to the potential even at small concentrations; the suspension is flocculated in the absence of lecithin, but becomes dispersed at lecithin concentrations above about 10%. Although our understanding of electrophoresis in non-aqueous systems is still primitive, such studies allow at least an empirical understanding of stability and surfactant adsorption in these systems.

Figure 7. Demonstration of how an ionic surfactant can affect zeta potential.

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information please visit Malvern Panalytical.