Milk is a key source of nutrients for both children and adults. With its high importance, milk is available in various forms: fresh, powdered, boxed (ultra-heat treated), and evaporated. The most widely consumed form of milk varies worldwide, based on a number of factors, including culture, geography and climate.
Analyzing the nutrients in milk is important when monitoring milk quality. Micronutrients can be present in milk naturally or added to fortify the milk either to meet market demands or regulatory requirements.
With increasing regulatory oversight and the mandatory inclusion of nutrients, organizations explore options to prevent systemic malnutrition and ensure that milk is unadulterated. People are also responding by demanding micronutrient monitoring to improve milk quality and by choosing fortified products over non-fortified products in the marketplace.
Internal quality control and the possibility of external monitoring provide strong incentives for milk producers to rapidly, easily, and accurately monitor nutrients in their products. Accurately assessing nutrients is also a requirement outlined in nutritional labeling guidelines to meet regulatory compliance.
With a large dynamic range, robust operating conditions, and rapid multi-element throughput, inductively coupled plasma optical emission spectroscopy (ICP-OES) is the technique of choice in a multi-element analytical environment with detection capabilities suitable for nutritional analysis.
Flame atomic absorption (AA) systems can be an attractive alternative solution, due to advantages such as simplicity, cost savings, and single-element analytical speed.1 However, the downside of this method is that each sample has to be analyzed individually for each element for measuring multiple elements, eliminating its speed advantage for multi-element analysis.
This article discusses the analysis of micronutrient content in various commercial milk products using a PerkinElmer Avio™ 200 ICP-OES. Here, a PerkinElmer Titan MPS™ Microwave Sample Preparation System is used to prepare the samples.
Experimental
Samples and Sample Preparation
Samples were purchased from local markets and were chosen to be representative of common forms of milk products available, including both fresh and non-perishable. The samples studied were representative of whole, non-fat, and reduced-fat forms of fresh, boxed, evaporated, and powdered milk as well as the NIST™ SRM 1549a Whole Milk Powder. The samples were analyzed for commonly found nutrients in milk and milk products.
A PerkinElmer Titan MPS microwave digestion system was used to prepare the milk samples for analysis by closed-vessel microwave-assisted digestion. Tables 1 and 2 list the digestion method, reagents, and sample parameters. After weighing and placing the samples into the digestion vessels, the reagents and any sample spikes were added.
The reagents and samples were allowed to sit open in the vessels for 10 minutes so that any early reactions can occur safely before being sealed and placed into the Titan MPS for digestion. After completing the digestion process, the samples were transferred out of the digestion vessels by triple-rinsing with deionized (DI) water into sample vials for subsequent analysis.
Table 1. Titan MPS digestion method.
| Step |
Target Temp (°C) |
Pressure Limit (bar) |
Ramp Time (min) |
Hold Time (min) |
Power Limit (%) |
| 1 |
140 |
35 |
10 |
2 |
80 |
| 2 |
195 |
35 |
3 |
20 |
100 |
| 3 |
50 |
35 |
1 |
20 |
0 |
Table 2. Digestion information.
| Parameter |
Volume |
| Reagents Used |
2.5 mL of HNO3 (70%)
+ 7.5 mL deionized water |
| Initial Sample Weight |
1 g |
| Final Solution Volume (after dilution) |
50 mL |
Instrumental Conditions
An Avio 200 ICP Optical Emission Spectrometer was used to perform all analyses. Tables 3 and 4 outline the target elements and instrument conditions for the analysis. The standard sample introduction system featuring a Meinhard® glass nebulizer and baffled glass cyclonic spray chamber was used.
Each element was analyzed using an auto integration range of 0.1 – 5 seconds. With this wide range, the most appropriate integration time can be automatically determined by the Avio 200 spectrometer for each element.
A longer integration time was used for lower concentration elements and a shorter time for higher concentration analytes. This capability shortens sample analysis time, and in combination with the low argon consumption (8 L/min), results in a significant saving when the cost of argon is considered.
External calibration standards were prepared in 5% nitric acid (v/v) from two single-element standards and a multi-element stock at the concentrations listed in Table 5. The calibration standards were prepared in two ranges so that maximum accuracy can be achieved for both low- and high-level elements.
The final nitric acid concentration of the standards (5%) was selected to match the acid concentration of the digested and diluted samples. Yttrium (Y) was added at 0.5 ppm as an internal standard to all solutions.
Table 3. Avio 200 ICP-OES instrumental parameters.
| Parameter |
Value |
| Nebulizer |
Meinhard Glass, Type K1 (Part No. N0777707) |
| Spray Chamber |
Baffled Glass Cyclonic (Part No. N0791352) |
| Sample Uptake Rate (mL/min) |
1.0 |
| RF Power (W) |
1500 |
| Nebulizer Gas (L/min) |
0.70 |
| Auxiliary Gas (L/min) |
0.2 |
| Plasma Gas (L/min) |
8 |
Table 4. Method parameters.
| Element |
Wavelength (nm) |
Plasma View |
Points per Peak |
Auto Integration Range (sec) |
| Ba |
455.403 |
Radial |
3 |
0.1 – 5 |
| Ca |
317.933 |
Radial |
3 |
0.1 – 5 |
| Fe |
238.204 |
Axial |
3 |
0.1 – 5 |
| K |
766.490 |
Radial |
3 |
0.1 – 5 |
| Mg |
285.213 |
Radial |
3 |
0.1 – 5 |
| Na |
589.592 |
Radial |
3 |
0.1 – 5 |
| P |
178.221 |
Axial |
3 |
0.1 – 5 |
| S |
181.975 |
Axial |
3 |
0.1 – 5 |
| Sr |
407.771 |
Radial |
3 |
0.1 – 5 |
| Zn |
206.200 |
Axial |
3 |
0.1 – 5 |
| Y (int Std) |
371.029 |
Axial & Radial |
3 |
0.1 – 5 |
Table 5. Calibration standards.
| Element |
Std 1 (µg/L) |
Std 2 (µg/L) |
Std 3 (mg/L) |
Std 4 (mg/L) |
| Ba, Fe, Sr, Zn |
50 |
100 |
---- |
---- |
| Ca, K, Mg, Na, P, S |
---- |
---- |
1 |
10 |
Results and Discussion
Although the milk samples vary in terms of form, density and fat content, the samples were easily and rapidly prepared for analysis by the Titan MPS digestion system using a minimum volume of reagents in a much shorter time compared to open-vessel digestions. Clear solutions were yielded by all digests, indicating a complete digestion.
The accuracy of the method was established by analyzing the NIST™ 1549a Whole Milk Powder (Table 6). Despite being a very challenging sample to analyze due to its high fat content in a concentrated form, all recoveries were within 10% of the certified values, highlighting the accuracy of the methodology and corroborating the capability of the Avio 200 ICP-OES to determine large variations of elemental concentrations in one analysis.
After establishing the accuracy, the other milk samples were analyzed (Figure 1). As expected, the powdered milks had the highest elemental concentrations, followed by the evaporated milks, boxed, and fresh milks, where the elemental concentration was the lowest.
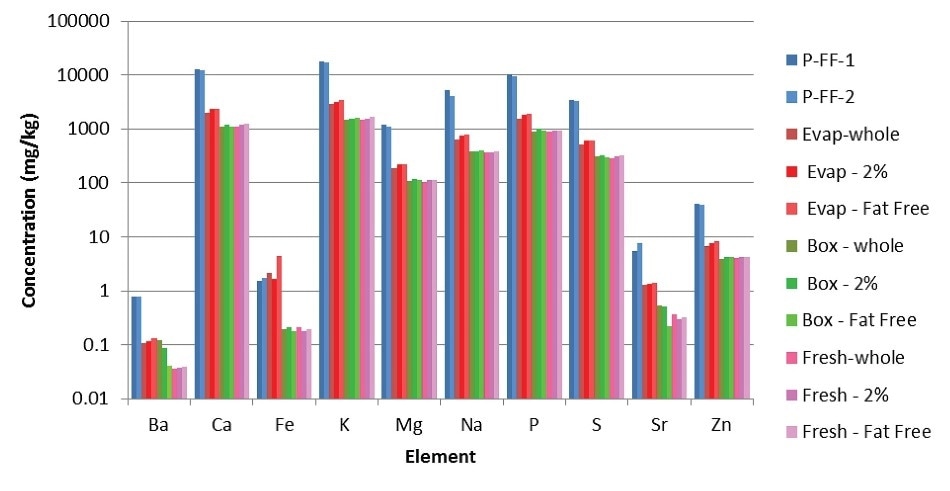
Figure 1. Results from analyses of milk samples (powdered milks in shades of blue; evaporated milks in shades of red; boxed milks in shades of green; fresh milks in shades of pink).
Table 6. Analysis of NIST 1549a whole milk powder.
| Element |
Experimental (mg/kg) |
Certified (mg/kg) |
% Recovery |
| Ba |
0.530 |
0.566 |
94 |
| Ca |
9203 |
8810 |
104 |
| Fe* |
1.72 |
1.8 |
95 |
| K |
11920 |
11920 |
100 |
| Mg |
845 |
892 |
95 |
| Na |
3185 |
3176 |
100 |
| P |
7128 |
7600 |
94 |
| S |
2239 |
--- |
--- |
| Sr |
2.04 |
2.14 |
95 |
| Zn |
33.7 |
33.8 |
100 |
* Reference value
Each sample type (fresh, boxed, evaporated or powdered) has consistent elemental concentrations irrespective of its fat content. Also, nutrients in the boxed milks are at the same level of fresh milks, indicating that the nutritional quality is not degraded by the ultra-heat treatment of the boxed milks. This treatment is needed to keep the boxed milks stable without the need for refrigeration.
The results also reveal the value of milk as a food source. Milk contains elevated levels of nutrients such as sodium, magnesium, potassium, and calcium, along with sulfur and phosphorus.
Custom dilutions for each element were not required due to the availability of large dynamic range and the Dual View capability of the Avio 200 ICP-OES. With two levels of calibration standards (Table 5), it is possible to analyze all elements in a single analytical pass per sample.
Any remaining matrix effects from the different samples were assessed by spiking all of the samples before digestion with all elements at the levels listed in Table 7. These spike levels represent the concentrations in solution after sample preparation and are slightly more than the concentrations of the unspiked milks.
This condition ensures that the spike level is a valuable parameter with regard to the sample signal for the purposes of analytical evaluation. The powdered milk samples were not spiked as analysis of the NIST™ milk powder showed the absence of matrix effects.
Figure 2 shows the resulting spike recoveries and all fall within 10% of the spiked value. Achieving effective digestion with the Titan MPS system eliminated the need for per-sample matrix-matching to obtain remarkable spike recovery.
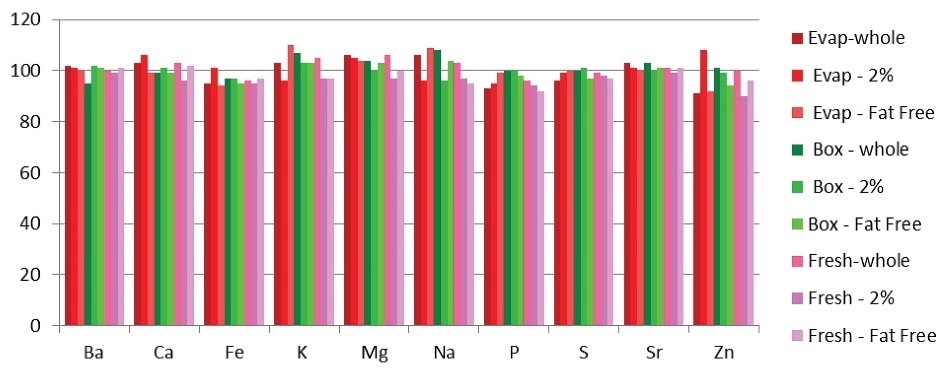
Figure 2. Spike recoveries in milk samples (evaporated milks in shades of red; boxed milks in shades of green; fresh milks in shades of pink).
Table 7. Pre-digestion spike levels.
| Element |
Spike Level |
Units |
| Ba |
10 |
µg/L |
| Fe, Sr, Zn |
100 |
µg/L |
| Mg |
5 |
mg/L |
| Na, S |
15 |
mg/L |
| Ca, K, P |
50 |
mg/L |
Conclusion
This article has shown the capability of the Avio 200 ICP-OES to analyze a range of milk samples reliably and effectively for multiple elements over a broad range of concentrations. Thanks to its extended capabilities, the spectrometer delivers higher multi-element throughput compared to Flame AA, while enabling a simple elemental analysis of sulfur and phosphorus, which is typically a challenging task for Flame AA.
The use of the Titan MPS microwave digestion system not only simplifies sample preparation, but also increases productivity and throughput of the lab in comparison with hot plate or hot block digestions. The ability to achieve complete digestion of samples simplifies the analysis by eliminating the need for matrix-match calibration standards.
Nutritional elements present in milk can be rapidly, easily, and accurately analyzed using the combination of the Titan MPS digestion system for sample preparation and the Avio 200 ICP-OES for subsequent analysis.
Consumables Used
| Avio 200 ICP-OES |
| Component |
Part Number |
| Red/Red PVC Pump Tubing |
09908585 |
| Black/Black PVC Pump Tubing |
09908587 |
| Autosampler Tubes |
B0193233 (15 mL)
B0193234 (50 mL) |
| Instrument Calibration Standard 2 (100 mg/L) |
N9301721 (125 mL) |
| Pure-Grade Phosphorus Standard (1000 mg/L) |
N9303788 (125 mL)
N9300139 (500 mL) |
| Pure-Grade Sulfur Standard (1000 mg/L) |
N9303796 (125 mL)
N9300154 (500 mL) |
| Titan MPS Digestion System |
| Component |
Part Number |
Consumables Kit for Standard 75 mL
Digestion Vessels |
N3132000 |
Rupture Disks for Standard 75 mL
Digestion Vessels (25 pieces) |
N3132001 |
Pressure Seal for Standard 75 mL
Digestion Vessels (10 pieces) |
N3132002 |
| End Cap Plug for Gas Containment Manifold |
N3134004 |
| Single Lip Seal Forming Tool for Standard 75 mL Digestion Vessels |
N3132015 |
| 8-Position Lip Seal Forming Tool for Standard 75 mL Digestion Vessels |
N3132014 |
References
1. Spivey, Nick, “Analysis of Micronutrients in Milk by Flame Atomic Absorption Using FAST Flame Sample Automation for Increased Sample Throughput”, Application Note, PerkinElmer, 2015.

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer Inc.
For more information on this source, please visit PerkinElmer Inc.