Sponsored by Nanosurf AGReviewed by Olivia FrostJan 19 2023
Micron-sized particles are available commercially in a variety of materials, including polymers, (magnetic) metals, glass, and ceramics. They are used in biology, medicine, material science, and many engineering domains.
These particles are utilized for a variety of purposes, including medicine administration or timed release of drugs, modifying the viscosity of slurries in the casting industry, as filler materials, as contrast agents in e-displays, and the production of dentures.
The physical features of the particles, specifically their size distribution and density, are critical for controlling certain capabilities such as magnetic properties, packing density, or flow dynamics. Careful characterization of these beads’ surface area, composition, and size or mass is a critical factor in their function.
In this article, unprecedented mass/size measurements of micrometer-sized particles are presented by combining two cutting-edge technologies: PicoBalance and FluidFM®.
Specifically, hollow cantilevers that are commercially available (FluidFM probes) are employed to accurately aspirate, retain, and then release individual particles.
To determine the mass of the particles, a device called PicoBalance was used, a technology that detects the mass of beads by detecting the frequency shift of a resonating cantilever.
PicoBalance
PicoBalance is a cantilever-based technique for determining the mass of a sample connected to the free end of the cantilever (see Fig. 1). Typically, the particle is connected to a tipless cantilever, and the change in resonance frequency before and after attachment is measured to calculate the mass.1-3
To compute the mass, three parameters are necessary: (1) the change in resonance frequency (see Fig. 2), (2) the spring constant of a cantilever, and (3) the location of the connected mass on the cantilever.
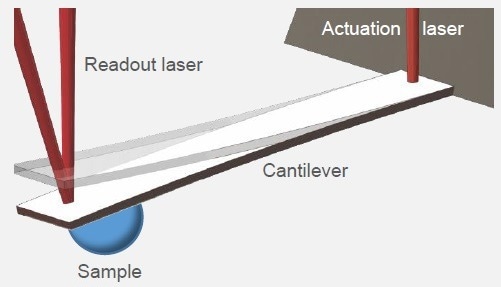
Figure 1. PicoBalanceschematic. The mass of a sample is measured by determining the mass-induced shift in resonance frequency of an oscillating cantilever. Actuation and readout are done via near-infrared lasers. Image Credit: Nanosurf AG
For precise mass measurements, a clean and reliable actuation of the cantilever is necessary. By acoustic (piezo) actuation, clean and consistent actuation is difficult, particularly in liquids, where a phenomenon known as the “forest of peaks”4,5 obscures the cantilever resonance.
To prevent this distortion, PicoBalance employs photothermal actuation (CleanDrive) of the cantilever, and the resultant textbook, like amplitude and phase response, permits precise monitoring of the resonance frequency.
The spring constant may be measured using multiple methods before the experiment if it has not previously been calibrated. In general, the best results are yielded using the Sader technique.6
This approach takes advantage of the planar dimensions of the cantilever. Its damping and resonance frequency are determined in the air using a heat spectrum or by actuating the cantilever.
As the mass sensitivity changes with orientation, the position of the connected masses must be taken into consideration. It can be determined optically; however, when FluidFM probes are used, it is well defined.
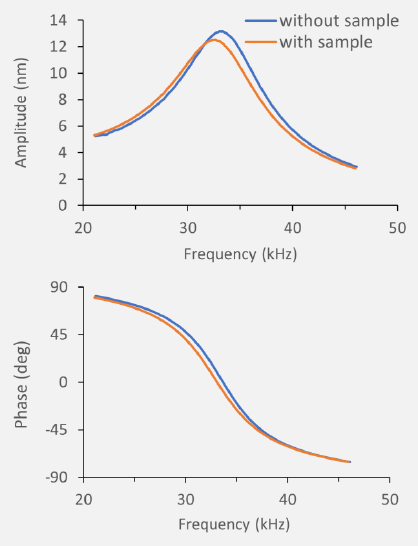
Figure 2. Attachment of a bead to the cantilever leads to a resonance frequency shift that allows the mass determination of the bead. Image Credit: Nanosurf AG
FluidFM®
The FluidFM technology is built on a hollow cantilever with an opening at the free end (Fig. 3). Using a reservoir that is operated by an accurate pump, the hollow cantilever functions like a micropipette.7 Particle adhesion is both fast and reversible.
In practice, the cantilever is brought within 10 µm of the particle that is to be attached, and a modest underpressure (- 50 mbar) is provided to suction-attach the particle onto the cantilever.
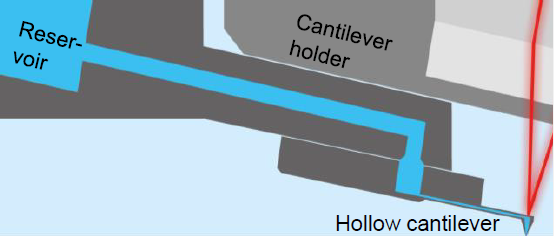
Figure 3. Schematic of FluidFM. A hollow FluidFM cantilever is connected to a pressurized fluid reservoir. Image Credit: Nanosurf AG
After the mass of the particle has been determined, an overpressure may be applied to free the particle. The location of the connected particle is known and reproducible, and it matches the location of the aperture.
In the example shown (Fig. 4), the aperture is 8 µm in diameter and 4.4 µm from the free end. Without FluidFM, the connection of particles is dependent on glue or chemical functionalization.
These procedures may cause inaccuracies in the particle mass, and the attachment procedure is irreversible, requiring a new cantilever for each experiment. Nevertheless, for certain biological samples, a chemical functionalization may be more suited.
Small beads are difficult to manage and collect in air or vacuum conditions due to strong electrostatic interactions. Working in liquids at the right pH (i.e., in their storage buffer solution), aggregation can be prevented, and FluidFM technology simply permits the choice of individual particles.
The lowest discernible change in the resonance frequency of the FluidFM probe was determined to be 15 Hz, corresponding to a mass resolution of 0.12 ng.
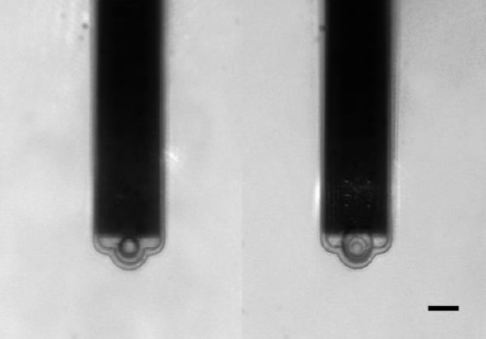
Figure 4. Left, 10 μm bead attached to FluidFM probe. Right, 15 μm bead attached to FluidFM probe. Scale bar is 15 μm. Image Credit: Nanosurf AG
Mass Distribution of Silicon Dioxide and Polystyrene Beads
As a physical model, silicon dioxide (SiO2) beads with notional diameters of 10, 11, 13, 14, and 15 µm and polystyrene beads (PS) with diameters of 10 and 15 µm were determined utilizing PicoBalance with FluidFM probes. Twenty-five separate examples of each kind of bead were taken sequentially.
The outcomes of all 175 tests are shown in Figure 5. The predicted mass (x-axis) and uncertainty were calculated using the manufacturer’s specifications for the density and nominal diameter.9,10
The masses discovered using the PicoBalance-FluidFM combo (y-axis) have a variance of only 0.1 ng. To increase this value, smaller cantilevers may also be employed.
The significant connection between the predicted and observed mass suggests that the frequency shift provides the total mass of the beads and does not measure the buoyant mass (mass of the bead minus the mass of the displaced liquid).
The measured mass matches the predicted mass based on the diameter of the bead and given density by the producer. Furthermore, the mass uncertainty given by the manufacturer specifications is greater than the uncertainty in the PicoBalance readings, indicating that the cantilever-based mass sensing approach is more precise.
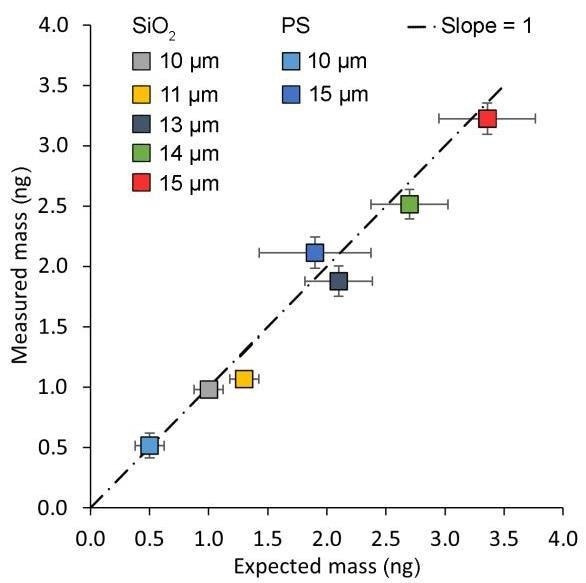
Figure 5. Results of 175 mass measurements on different beads. The uncertainty as represented by the error bars is lower than expected from the manufacturer's specifications. A straight line of slope 1 indicates the expected behavior for an accurate, linear measurement. n=25 per data point. Image Credit: Nanosurf AG
Summary
The integration of FluidFM and PicoBalance technologies allows for exact mass estimates of colloidal beads in solution and provides a user-friendly framework for particle separation and distribution.
PicoBalance, which is enabled by photothermal excitation (CleanDrive), is a precise approach for determining the total bead mass. Using the FluidFM technology, the measuring technique is rapid and highly reproducible.
The PicoBalance is the only commercially available method for determining the overall mass of samples on the scale of picograms to nanograms.
Other non-commercial cantilever-based mass measurement systems have one of two disadvantages: they measure just the buoyant mass (total mass minus the mass of the sample displaced liquid) or have inferior mass and temporal resolution. They also lack concurrently obtained, supporting optical data.
References
- Martinez-Martin, D., Fläschner, G., Gaub, B. et al. Nature 550, 500-505 (2017).
- Fläschner, G., Roman, C. I., Strohmeyer, N. et. al. Nat Commun 12, 2922 (2021).
- Cleveland, J. P., Manne, S., Bocek, D. & Hansma, P. K. Rev Sci Instrum 64, 403-405 (1993).
- Nanosurf. CleanDrive: Photothermal Excitation of the Cantilever (2021).
- Carrasco, C., Ares, P., de Pablo, P. J. & Gomez-Herrero, J. Rev Sci Instrum 79, 126106 (2008).
- Sader, J. E., Larson, I., Mulvaney, P. & White, L. R. Rev Sci Instrum 66, 3789-3798 (1995).
- Meister, A., Gabi, M., Behr, P. et al. Nano Lett 9, 2501-2507 (2009).
- Gunstheimer, H. Improved mass calibration of an intertial pico-balance using microparticles, TU Ilmenau (2021).
- MicroParticles GmbH (2021).
- Polysciences Inc. (2021).

This information has been sourced, reviewed and adapted from materials provided by Nanosurf AG.
For more information on this source, please visit Nanosurf AG.