Sponsored by Nanosurf AGReviewed by Olivia FrostApr 3 2023
The study of biological systems varies from whole organisms, organs, and organoids, down to their building blocks of proteins and cells.
At the lower end of the scale, atomic force microscope (AFM) systems are utilized in multiple disciplines of biological research, such as single-cell manipulation, imaging of bacteria and live cells, or force spectroscopy on molecules, cells, and tissue.
This article will discuss advanced biological experiments on cells, as well as how particular accessories can be utilized to conduct such experiments.
Combining Bio-AFM with Optical Microscopy
Bio-AFM is especially powerful when combined with other methods, particularly optical microscopy. Nanosurf provides stages for all popular brands of inverted microscopes for this reason.
These stages are attached to the body of the microscope, enabling the AFM to be placed atop the bio-sample between the objective and condenser.

Image Credit: Nanosurf AG
Establishing Physiological Conditions
Mammalian cells are prominent samples for Bio-AFM experiments. These experiments are frequently performed at room temperature with a CO2-independent, pH-stabilizing buffer. These non-physiological conditions may be practical and effective for answering some scientific problems, but they are not optimal.
They prevent long-term experiments (those that exceed four hours), as cell deterioration is visible. Moreover, the validity of some scientific inferences is uncertain, even for short time frames.
It has been shown that buffer conditions affect ion channel conductance,1 molecule update,2 can inflict phototoxicity3 and change cell stiffness,4 whereas the temperature affects characteristics such as cell stiffness4 and adhesion measurements.5
Nanosurf provides accessories such as the AFM-compatible Live Cell Incubator to control humidity, temperature, and CO2, allowing for physiological conditions in an experiment.
Controlling the Temperature
The Petri dish holder accommodates glass and plastic bottom dishes up to 10 mm in height and 50 mm in diameter. It may be utilized in standalone applications or together with optical light microscopy.
The holder has heating elements that, in combination with the TEC controller, enable the temperature of the sample to be controlled, ranging from ambient to 60 °C. The temperature is able to be held constant within 0.1 °C.
Ideal proliferation can be ensured for many cells when a temperature of 37 °C is maintained.
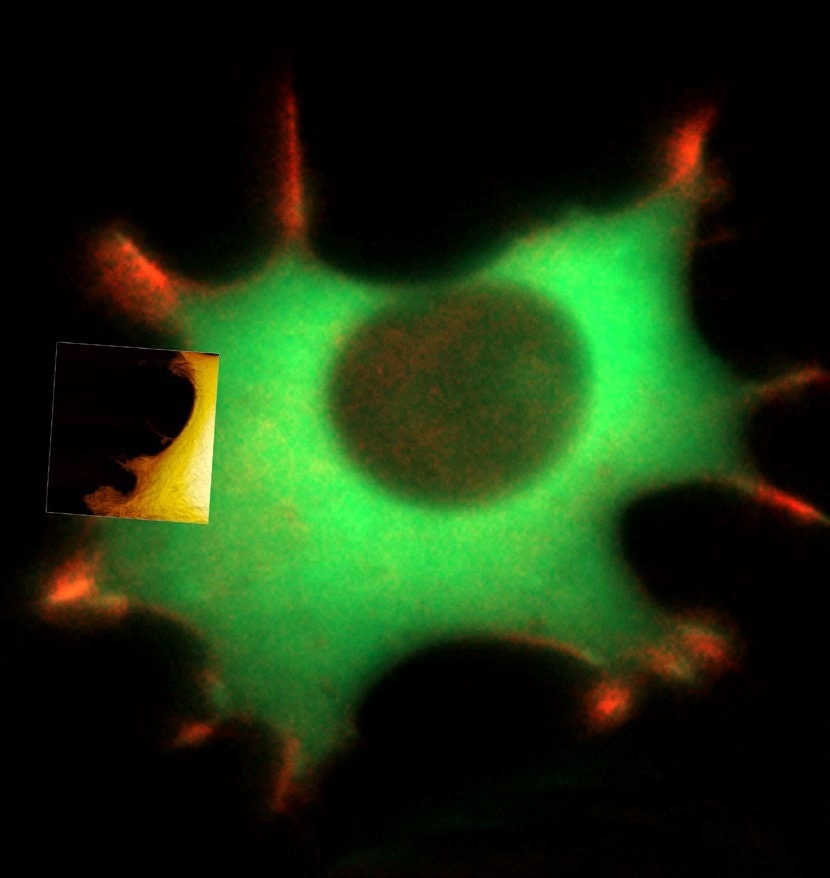
Figure 1. Live fibroblast in cell culture conditions imaged with inverted light microscope and AFM (overlay). Bottom shows AFM image of the cell with genetically encoded fluorescent makers (actin-mcherry, gfp-paxilin). AFM image, 10 μm width. Image Credit: Nanosurf AG
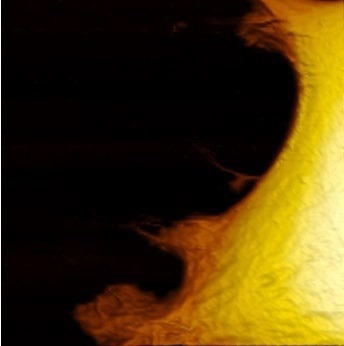
Image Credit: Nanosurf AG
For temperature-critical experiments, local heating via the Petri dish holder may not be sufficient as a temperature gradient between the edge of the Petri dish and the center is difficult to prevent.
A heating option that elevates the temperature around the complete optical microscope and AFM is available to reduce temperature gradients in the Petri dish. If the AFM is placed in an acoustic enclosure, heating can be applied to its complete volume.
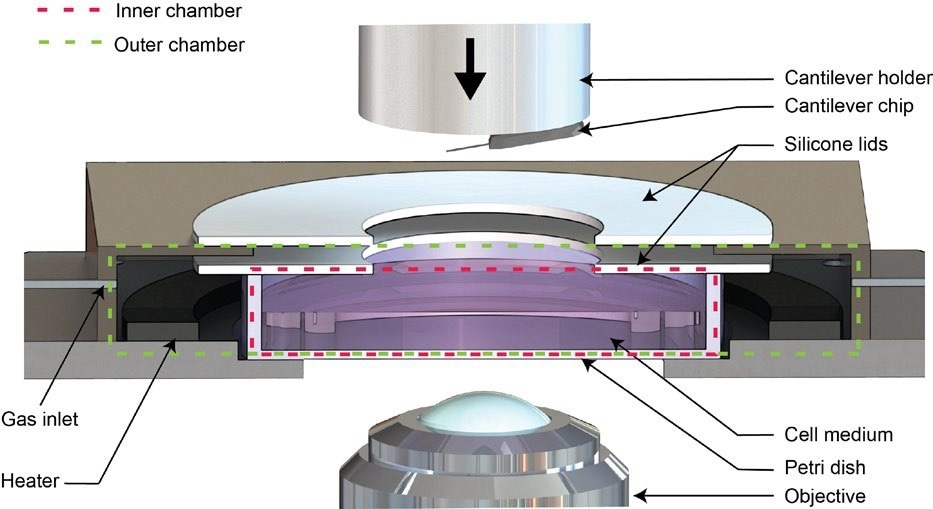
Figure 2. Schematic of the Live Cell Incubator. Image Credit: Nanosurf AG
For long-term experiments,6 a global acoustic enclosure heater was used in conjunction with a local Petri dish heater. The global heating targeted 35 °C, which is just below the target temperature of the Petri dish heater. This merges small gradients with a quick response time after mounting a new sample or cantilever.
Motorization of the AFM, stage, and optical microscope mitigates the requirement to open the acoustic enclosure to identify areas of interest, align the laser, or change the optical magnification or fluorescence filter set.
The increased temperature also increases the evaporation of the medium. To resolve this issue while also controlling the pH of CO2-dependent cell culture media, Nanosurf provides the option of a Live Cell Incubator.
Controlling Humidity and pH
The Live Cell Incubator option is a modification to the standard Petri dish holder. It seals the Petri dish, creating two chambers while allowing the cantilever holder to move freely. The inner chamber consists of the closed Petri dish, while the outer chamber encloses the Petri dish (see Figure 2).7

Figure 3. Identical cell at t = 0 and t = 12 h during cell mass measurement (PicoBalance) facilitated by the Live Cell Incubator. Image Credit: Nanosurf AG
By combining the Live Cell Incubator with a suitable supply of humid gas, evaporation can be reduced to a minimum. The basic approach is to use a washing bottle to humidify bottled gas. In practice, this may be synthetic air with 5% CO2, allowing pH control in CO2-dependent cell culture media (e.g., DMEM).
Nanosurf also provides an advanced humidification scheme that controls the humidity, from ambient to 95% rel. hum. Via a humidity sensor and microcontroller.
If so desired, Nanosurf also offers a solution without the need for bottled gas, and the mixing of air with CO2 is done in situ using line gas.
The experimental conditions can be kept the same as the cell culture in the fashion described above. The correct temperature, low evaporation and pH control not only ensure relevant insights into near physiological systems but also unlock experiments that unfold over the course of a few days, for instance, when researching tissue formation6 with the PicoBalance.
Table 1. Humidification schemes overview. Source: Nanosurf AG
| Humidification schemes |
| Basic |
Advanced |
Pro |
Bottled gas,
≈90% rel. hum. |
Bottled gas,
ambient to 95% rel. hum. |
Contr. gas mixing,
ambient to 95% rel. hum. |

Figure 4. Fluid inside the Petri dish can be added or removed using the perfusion insert. Image Credit: Nanosurf AG
Perfusion of Cell Media
Despite the importance of studying cells under physiological conditions, research also requires disturbing the cells in a controlled fashion, e.g., by changing media.
An additional option to the Petri dish holder is Nanosurf’s perfusion-insert (see Figure 4). With the AFM atop the Petri dish, ideally enclosed by the Live Cell Incubator, adding chemicals to the Petri dish or switching media is more difficult.
If an experiment is to run autonomously, or a specific area of the dish requires revisitation, fluid insertion or fluid exchange must be feasible without human intervention.
To enable this, the perfusion insert consists of a ring, onto which two bent tubes are magnetically attached and connected to flexible tubing. This tubing can then be connected to, e.g., syringes or pumps, which allow a fluid, or exchange media (push-pullmechanism) to be added.
Single Cell Force Spectroscopy
The 150 µm-stage is a Petri dish holder with integrated long-range piezo actuators, enabling the precise positioning of the dish surface in height (z-axis), increasing the z-range of the AFM. This is particularly interesting in the single cell force spectroscopy (SCFS) field but may also be utilized in other applications such as 3D printing.
In SCFS, cell adhesion may be differentiated down to the molecular level. For these experiments, cells may be attached to the cantilever using an adhesive protein and subsequently brought into contact with the dish. After an optional waiting time, they are retracted.
Even after the cell’s body is no longer in contact with the surface, adhesion events caused by tethers are recorded.7 A microscope objective positioner is also available.
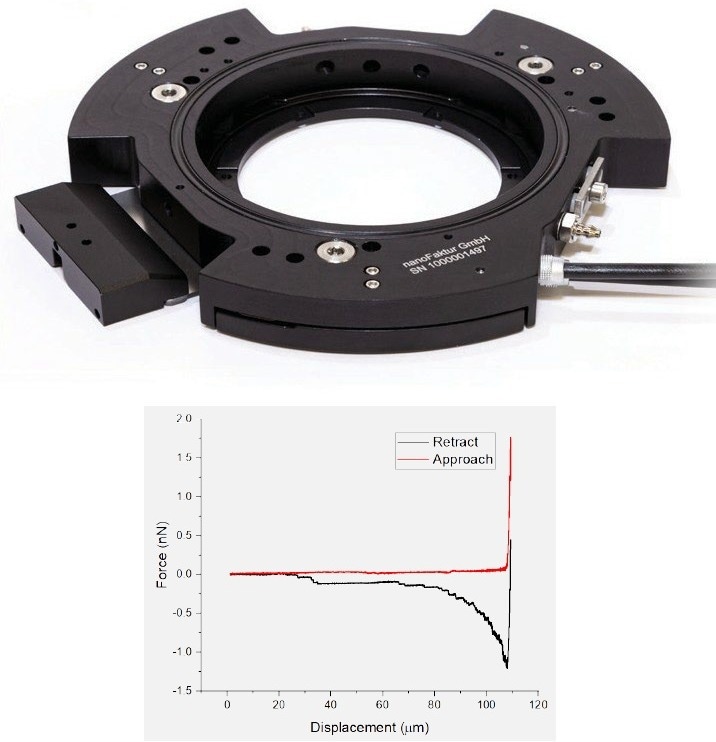
Figure 5. Petri dish holder with integrated piezo motors (top) enabling single cell force spectroscopy experiments (bottom). Image Credit: Nanosurf AG
Cell Manipulation via FluidFM®
FluidFM combines the functionality of both micro-pipettes and force-sensing cantilevers. This provides a unique opportunity for probing and manipulating cells.
The FluidFM probe is a cantilever with an embedded microfluidic channel and aperture at the free end. It facilitates partial aspiration of the cell using underpressure.
As the holding force between the cell and cantilever is significantly higher than using adhesive proteins alone, the adhesion of fully adherent cells may be probed. This also enables high throughput measurements of single-cell adhesion.9
Using FuidFM probes that are microsyringes instead of micropipettes extends the functionality of FluidFM even further. These micro-syringes can be used for targeted micro-injection of cells or, the reverse, the extraction of cell fluid,10 e.g., for the purpose of TEM, protein assays and PCR, or even single cell transcriptomics.11
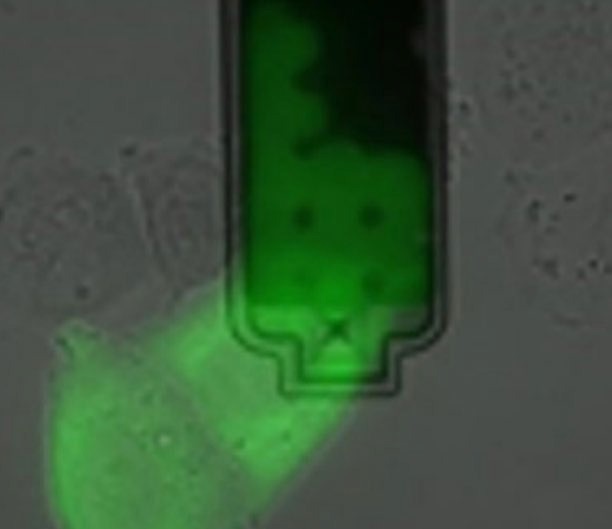
Figure 6. Extraction of sub-picoliter sample of nucleoplasm and cytoplasm from live cells without killing them. Image Credit: Nanosurf AG
Summary
Biological experiments frequently have a specific set of requirements. These may concern the establishment of a favorable, near-physiological environment in terms of humidity, temperature, and CO2.
Alternatively, these requirements may involve manipulation of the sample via the addition of chemicals, separating it from the dish surface.
By offering this wide range of accessories, in particular the different options for Petri dish holders (heater, Live Cell Incubator, perfusion-insert, and piezo-motors), Nanosurf provides researchers with the tools that they need to maximize the potential of AFM in biological applications.
List of Mentioned Accessories
The experiments described in this article were conducted with a DriveAFM on a Zeiss inverted optical microscope. The components and accessories utilized are a Petri dish holder with heating, a Live Cell Incubator, a perfusion insert, a 150-µm piezo stage, and FluidFM®.
Nanosurf’s DriveAFM is compatible with all accessories, while Nanosurf’s CoreAFM and FlexAFM are compatible with all accessories except the Live Cell Incubator.
References
- Hanrahan, J. W. & Tabcharani, J. A. (1990) Inhibition of an outwardly rectifying anion channel by HEPES and related buffers. J. Membr. Biol., 116.
- Otero, D. et. al. (1985) Effects of 4-(2- hydroxyethyl)-1-piperazine-ethanesulfonic acid (AH5183) on rat cortical synaptosome choline uptake, acetylcholine storage and release. Brain Res., 359.
- Lepe-Zuniga, J. L., Zigler, J. S., Jr & Gery, I. (1987) Toxicity of light-exposed Hepes media. J Immunol. Methods, 103.
- Chiou, Y-W. et. al. (2013) The Influence of Physical and Physiological Cues on Atomic Force Microscopy-Based Cell Stiffness Assessment. PLOS One, 8.
- Rico, F. et al. (2010) Temperature Modulation of Integrin-Mediated Cell Adhesion. Biophys J., 99.
- Martínez-Martín, D. et. al., (2017) Inertial picobalance reveals fast mass fluctuations in mammalian cells. Nature, 550.
- Martinez-Martin, D., Müller, D. J., Martin, S., Alsteens, D., Fläschner, G. (2016) WO2017012708A1.
- Sun, M., Graham, J. S., Hegedus, B., Marga, F., Zhang, Y., Forgacs, G. and Grandbois, M., (2005) Multiple membrane tethers probed by atomic force microscopy, Biophys. J., 89.
- Qiu, Y., et. al. (2022) Extending applications of AFM to fluidic AFM in single living cell studies. J. Cell Physiol., 237.
- Guillaume-Gentil, O. et. al., (2016) Tunable Single-Cell Extraction for Molecular Analyses. Cell, 116.
- Chen, W. et. al., (2022) Live-seq enables temporal transcriptomic recording of single cells. Nature, 608.

This information has been sourced, reviewed and adapted from materials provided by Nanosurf AG.
For more information on this source, please visit Nanosurf AG.