A study conducted by Carnegie Mellon University in collaboration with the Beatson Institute for Cancer Research has described how tiny molecular protein motors save energy or fold in on themselves when their transport services are not needed.
According to the research team, the answer to this molecular riddle provides new outlook into how molecular motor proteins are controlled and this may pave the way for treating numerous neurodegenerative diseases, such as Huntington's and Alzheimer's.
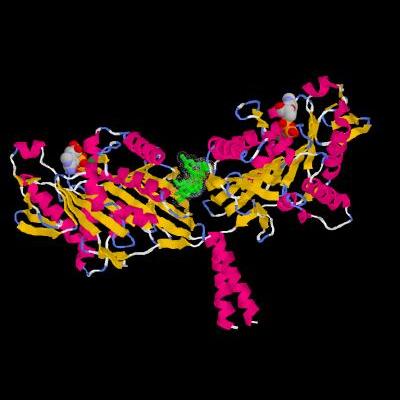 "Structural rendering of kinesin’s two heads, called motor domains, cross-linked by a bound tail domain (green). Credit: Carnegie Mellon University
"Structural rendering of kinesin’s two heads, called motor domains, cross-linked by a bound tail domain (green). Credit: Carnegie Mellon University
Hackney, professor of Biological Sciences in the Mellon College of Science, in his research focuses on kinesin-1, the key motor protein that causes the movement of cargo from the nerve cell body towards the axon. He collaborated with Frank Kozielski and Hung Yi Kristal Kaan at the Beatson Institute for Cancer Research in Glasgow, Scotland and crystallized a main part of the kinesin molecule, a tail bounded to the heads. The crystal structure observed proved that the complex consisted of only one tail and two head domains. Hackney performed biochemical manipulations to identify accurately the interaction of the tail with the heads, which resulted in a so called "double lockdown."
Kinesin's heads are commonly united at one point known as the hinge. In this new structure, the heads move in towards each other and are connected by the tail domain, successfully making the heads to cross-link at the tail binding site. This double lockdown occurring at the bridge and hinge avoids the separation of the heads. Since the heads are separated from each other in order to break down ATP, the double lockdown successfully prevents the generation of fuel by the molecule for powering the motor.
The scientists suggest that the same autoinhibitory mechanism may be used to regulate other kinesins. The kinesins also participate in chromosomal separation during the process of cell division, rendering the motors a target for tumor therapies that aims to inhibit the motors from choromosome transport resulting in prevention of cancer cells multiplication.