A team of scientists from the School of Arts and Sciences and School of Engineering at Tufts University has found a new way to produce a selective hydrogenation catalyst through individual atoms.
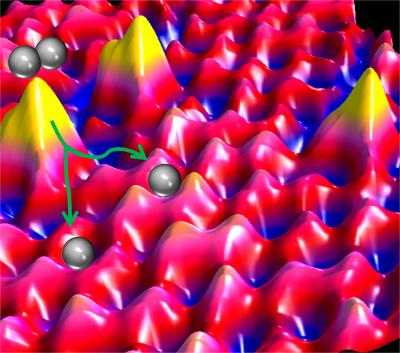 A team of researchers at Tufts University's School of Arts and Sciences and School of Engineering have discovered that individual atoms can catalyze industrially important chemical reactions such as the hydrogenation of acetylene, a development with potentially significant economic and environmental benefits. The team found that individual atoms of costly palladium (yellow peaks) when placed in the surface of copper metal (pink) help break apart hydrogen molecules (grey circles) into atoms, facilitating important chemical reactions. These single atom alloys save money because they use less precious metal than conventional catalysts. They also yield less chemical byproduct waste, so they are better for the environment. The research was published online and in print March 9, 2012 in Science (Image/Figure courtesy of Sykes Laboratory-Tufts University). Credit: Image/Figure courtesy of Sykes Laboratory-Tufts University
A team of researchers at Tufts University's School of Arts and Sciences and School of Engineering have discovered that individual atoms can catalyze industrially important chemical reactions such as the hydrogenation of acetylene, a development with potentially significant economic and environmental benefits. The team found that individual atoms of costly palladium (yellow peaks) when placed in the surface of copper metal (pink) help break apart hydrogen molecules (grey circles) into atoms, facilitating important chemical reactions. These single atom alloys save money because they use less precious metal than conventional catalysts. They also yield less chemical byproduct waste, so they are better for the environment. The research was published online and in print March 9, 2012 in Science (Image/Figure courtesy of Sykes Laboratory-Tufts University). Credit: Image/Figure courtesy of Sykes Laboratory-Tufts University
This catalyst offers significant environmental and economic benefits. The team has reported its research in the March 9 edition of Science.
Hydrogenation is critical to the pharmaceutical, petrochemical and food industries. It requires a catalyst, normally an alloy or a metal, that enables the hydrogen atoms to bind up with other molecules. According to the chemical engineers and chemists of Tufts, when individual atoms of palladium were added to copper, the single atom alloy that resulted from the process became selective and active for hydrogenation reactions.
E. Charles H. Sykes, senior author on the research paper and associate professor of chemistry at Tufts, reported that this research directly relates the grouping of single atoms in a metal alloy to their potency to catalyze hydrogenation reactions.
Industrial processes utilize small groups of precious metal with 5 to 10 nm wide on supports to create a catalyst. The researchers scattered individual atoms of palladium measuring below half a nanometer wide upon a copper support.
The scientists, during their research work, heated smaller amounts of palladium to nearly 1,000°C and at that temperature single atoms vaporized and enclosed themselves on the copper surface approximately three inches away. The Tufts team, through a scanning tunneling microscope, saw how these individual atoms scattered in the copper and how molecular hydrogen managed to separate at single, isolated palladium sites and scatter upon the copper surface layer.
Sykes stated that chemical reactions with small amounts of palladium utilize less energy and let go limited chemical byproduct waste, and so they are suitable for environment. He also said that the researchers, for the first time, have identified a precise microscopic picture of the grouping of atoms that support a catalytic hydrogenation reaction.
Mass spectrometry revealed that the new alloy separated the hydrogenation of both acetylene and styrene with more than 95% selectivity.