New methodologies for increasing catalytic hydrogenation performance using Pd-based catalysts are examined in a new study published in the journal ACS Omega.

Study: Recent Progress in Pd-Based Nanocatalysts for Selective Hydrogenation. Image Credit: ImageFlow/Shutterstock.com
Conventional Catalysis History
Catalysis in the twentieth century was mainly concerned with increasing activity and high turnover to produce more particles per unit time.
Meanwhile, due to higher processing costs and the negative environmental impacts of unpleasant byproducts, the current focus and strategic development aim to achieve good selectivity in all catalyst-based chemical reactions.
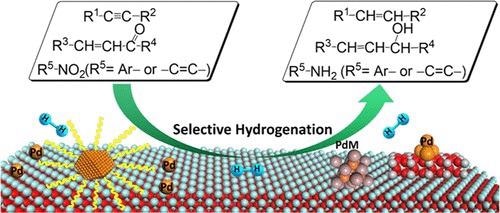
Selective hydrogenation of unsaturated bonds by palladium-based catalysts. © Zhao, X., et al. (2021).
Waste and depletion of resources have compelled the polymerization of many artificial chemicals to fulfill people's needs as communities have adapted. This situation necessitates improved catalyst selectivity and reduced byproduct creation in conformance with green chemistry fundamentals.
Hydrogenation Manufacturing
Many manufacturing processes rely on hydrogenation. To address low-carbon protection of the environment, efficient hydrogenation is extremely crucial, where only one intended functional group is restricted while other functional groups in the material remain unsaturated.
Fine chemical synthesis can be preferentially reduced by cheap and clean H2 to their correlating alcohols, alkenes, and amines, key intermediates for specialty chemicals, agrochemicals, polymeric materials, and pharmaceutical drugs.
The hydrogenation of acetylene and propyne in crude oil cracking gases and converting alkynes to olefins can be accomplished by selectively hydrogenating C to C to produce high-purity eth and propylene.
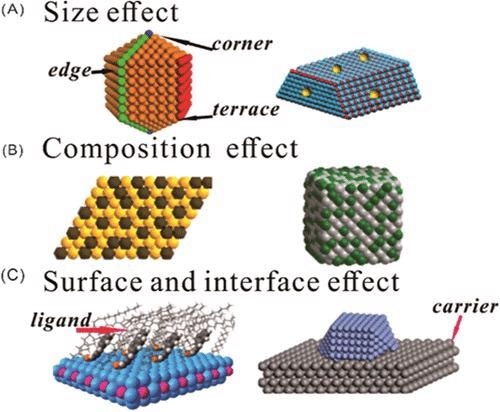
(A) Size effect, (B) composition effect, and (C) surface and interface effect over Pd-based catalysts. © Zhao, X., et al. (2021).
Hydrogenation with Pd Catalyst
An appropriate catalyst must be selected to obtain the desired products to attain specific hydrogenation. Pd-based catalysts are widely used during metal-organic chemical reactions. They perform admirably in a wide range of organic chemistry reactions, including selective hydrogenation, oxidative dehydrogenation, coupling, and cycloaddition.
Pd-based catalysts are also broadly used in selective hydrogenation due to their exceptional electronic configuration and capacity to absorb and activate hydrogen and unsaturated substrates. In recent decades, numerous attempts have been devoted to developing extremely selective and active Pd-based catalysts for selective hydrogenation.
Pd based Catalyst Advantage
Because of its excellent catalytic achievement, the precious metal palladium (Pd) is extensively used in metal-organic chemical reactions. This is primarily due to Pd's unfilled orbitals, which readily desorbed reactants on its surface. Pd0 has been thoroughly researched for chemical synthesis predicated on the nucleophilic addition of Pd0 to different organic clusters. Pd1+ and Pd2+ are excellent electrophiles that are air-stable and it can be dissolved in a wide range of organic solvents.
Pd2+ exhibits strong electrophilicity toward electron-rich substances including alkenes and alkynes. Pd1+ and Pd2+ substances can react to alkenes or alkynes to form unsteady intermediates, which can then be attacked and transferred by nucleophilic reagents to catalyze reactions. Because of these properties, Pd is widely used in catalytic reactions.
Limitation of Pd-based Catalyst
It is strongly suggested that Pd-based catalysts play an important role in selective hydrogenation reactions. Meanwhile, the exclusive summarization of the magnitude, composition, outer layer, interface effect, and surface or interfacial effect of metal Pd, which might significantly impact the function for selective hydrogenation, is still lacking.
Furthermore, there is no thorough review of the significant developments of various selective hydrogenation reactions over Pd-based electrocatalyst.
Improvement on Pd Catalyst
As catalysts, Pd nanoparticles are frequently equipped with supports. Using a carrier enhances Pd-based catalyst dispersion, alters Pd's electrical properties and distribution pattern, and forms strong metal–support interactions. This adjusts the arrangement and coordination configuration of the catalyst's particle surface, allowing selective adsorption of functional groups from the substrate and changing the adsorption energy of the material and intermediate.
A catalyst's composition alters its absorbency for substrates and intermediates, resulting in different catalytic performances and selectivities. The addition of other metals to Pd catalysts keeps costs down while also altering the electronic properties of the surface particle coordination structure caused by changes in the chemical structure of the material. A catalyst's selective hydrogenation capacity can be enhanced through synergic and electronic effects.
To achieve high chemical selectivity, the catalytically active sites must have consistent geometric and electrical structures, allowing the objective functional groups also to be adsorbed on the catalyst while avoiding numerous absorption mechanisms. As a result, modifying Pd-based catalysts' geometric and electronic configurations and monitoring their size help enhance their catalytic sensitivity and selectivity.
Further Research
Next research can determine the structure–activity correlation of Pd-based catalysts and create Pd catalysts of increased activity, selectivity, and stability. Numerous metal combinations can be used to obtain higher ethylene conversion and selectivity. This will be attributed primarily to the special binding sites.
Continue reading: The Applications of Nanoparticles as Synthetic Catalysts.
Reference
Zhao, X., et al. (2021). Recent Progress in Pd-Based Nanocatalysts for Selective Hydrogenation. ACS Omega. Available at: https://doi.org/10.1021/acsomega.1c06244
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.