A research team from the Stanford University has developed a simpler and faster technique to effectively modify the surface chemistry and significantly expand the surface area of nanowires with noble metal or metal oxide nanoparticles.
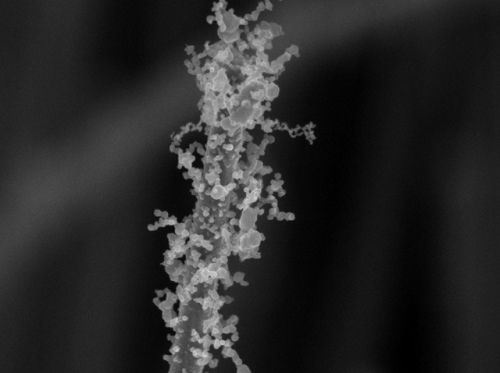 Decoration with nanoparticles creates intricate surface patterns full of nooks and crannies, twists and turns that greatly improve surface area. (Credit: Stanford Nanocharacterization Laboratory)
Decoration with nanoparticles creates intricate surface patterns full of nooks and crannies, twists and turns that greatly improve surface area. (Credit: Stanford Nanocharacterization Laboratory)
The findings of the study on bejeweled nanowires that resemble pipe cleaners have been reported in the Nano Letters journal. This novel technique paves the way to develop enhanced catalysts that produce new artificial fuels, high-efficient thin-film solar cells and superior lithium-ion batteries. The increased surface area of this decorated nanomaterial enables it to be used to detect trace amounts of chemicals such as toxins and explosives.
The research team used a quick burst of flame to adhere the nanoparticles to the nanowires. During the process, the nanowires were dipped in a solvent-based gel containing salt and metal and then air dried. The flame was then applied to the air-dried nanowires to burn out the solvent, enabling the crystallization of all-significant nanoparticles to form branch-like structures stemming out from the nanowires.
Xiaolin Zheng, senior author of the study, informed that the nanoparticle structure developed by the team increased the surface area of the nanowires several times more than the earlier attempts. The team named the process as the sol-flame method because of the use of a flame and solvent to synthesize the nanoparticle structures.
According to the team, the novel technique can be used with different nanoparticle and nanowire materials and offers a remarkable level of engineering control in synthesizing the nanoparticle decorations. The flame’s high temperature and short annealing time are the critical parameters in ensuring the homogeneous spread of the nanoparticles over the nanowires. The team can modify the size and density of the nanoparticle decorations by repeating the dip-coating process and by changing the nanoparticle concentration in the precursor solution.