Immunoassay is a biological test that can detect a host of diseases like cancer and Alzheimer’s. The detection of the disease is linked to the detection of biomarkers in the blood, saliva or urine sample taken from humans. Biomarkers are chemical representatives of diseases. The presence of biomarkers sets of fluorescence when subject to the immunoassay test. The intensity of the fluorescence is directly proportional to the amount of biomarkers.
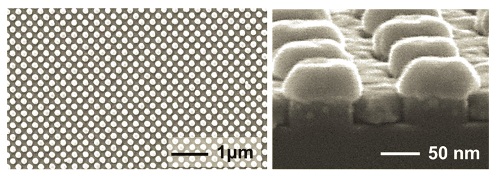 Princeton researchers dramatically improved the sensitivity of immunoassays, a common medical test, using the nanomaterial shown here. The material consists of a series of glass pillars in a layer of gold. Each pillar is speckled on its sides with gold dots and capped with a gold disk. Each pillar is just 60 nanometers in diameter, 1/1,000th the width of a human hair. Credit: Stephen Chou/Analytical Chemistry
Princeton researchers dramatically improved the sensitivity of immunoassays, a common medical test, using the nanomaterial shown here. The material consists of a series of glass pillars in a layer of gold. Each pillar is speckled on its sides with gold dots and capped with a gold disk. Each pillar is just 60 nanometers in diameter, 1/1,000th the width of a human hair. Credit: Stephen Chou/Analytical Chemistry
The current limitation to this important test is that faint traces of biomarkers produce very faint fluorescence that make it very difficult to detect the presence of diseases. Researchers at Princeton led by Stephen Chou, Professor of Engineering, have addressed this problem through a new nanomaterial D2PA that they have developed. D2PA comprises a glass pillar of 60 nm covering a thin layer of gold nanostructures. The pillars are each capped with a disk of gold with a smattering of gold nano-sized dots on the sides and spaced 200 nm apart. This structure has been shown to exhibit a 1 billion-fold increase in surface Raman scattering. Chou’s team replaced the traditional glass vials used in immunoassay tests with the new nanomaterial to greatly amplify fluorescence.
In current conventional immunoassay tests, the sample is added to glass vials containing antibodies that attach themselves to the biomarkers present in the sample. Another set of antibodies with attached fluorescent molecules are then added. The fluorescent antibodies are washed away if there are no biomarkers and if there are only few, the resulting fluorescence is very faint. The technology employed by Chou’s team produces a remarkably high enhancement of the fluorescent signal with very few biomarkers.
D2PA could find use in other areas as well since immunoassays are used for drug discovery too. They could also be applied to other areas of engineering and chemistry where fluorescence plays a significant role like solar energy harvesting and light-emitting displays.