Nov 5 2014
"On the one hand, ABC transporters causes diseases such as cystic fibrosis, while on the other hand they are responsible for the immune system recognising infected cells or cancer cells," explains Professor Robert Tampé from the Institute for Biochemistry at the Goethe University.
The considerable medical, industrial and economic significance of ABC transporters is also based on the fact that they cause bacteria and other pathogens to become resistant to antibiotics. Likewise, they can help cancer cells to defend themselves against cytostatic agents and thus determine whether chemotherapy will succeed.
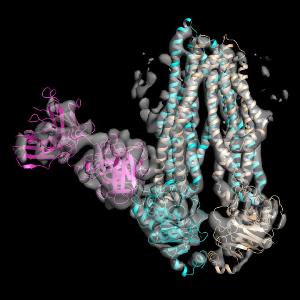 With the aid of a high-resolution cryo-electron microscope, the group led by Robert Tampé, in collaboration with colleagues at the University of California in San Francisco, succeeded in determining the structure of an asymmetrical ABC transporter complex. Credit: Robert Tampé
With the aid of a high-resolution cryo-electron microscope, the group led by Robert Tampé, in collaboration with colleagues at the University of California in San Francisco, succeeded in determining the structure of an asymmetrical ABC transporter complex. Credit: Robert Tampé
For the first time, the group led by Robert Tampé, in collaboration with colleagues at the University of California in San Francisco, succeeded in determining the structure of an asymmetrical ABC transporter complex with the aid of a high-resolution cryo-electron microscope. "Over a period of five years, we have successfully implemented a number of innovative, methodological developments. These have enabled us to gain insights that previously were unimaginable," says Tampé.
The researchers report in the current issue of the renowned scientific journal, Nature that they have succeeded in investigating a single frozen ABC transport complex at a subnanometer resolution that has never before been achieved. For this purpose, they used a newly developed single electron camera, new imaging processes and specific antibody fragments in order to determine the structure and conformation of the dynamic transport machine.
"The combination of physical, biotechnological, biochemical and structural biological methods has led to a quantum leap in the elucidation of the structure of macromolecular complexes," says Tampé. The method facilitates the targeted development of a trend-setting therapeutic approach.