May 13 2019
Earlier in 2016, the Faculty of Science and Technology at Aarhus University and Novo Nordisk entered into a strategic collaboration contract known as the ‘Aarhus Novo Nordisk Science and Talent Network.’
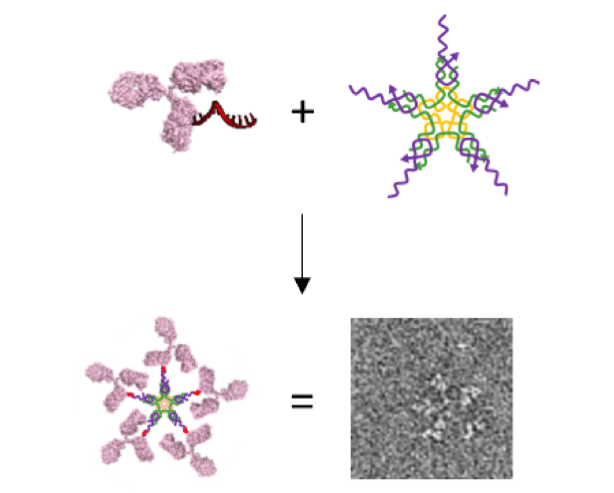 Collaboration between Novo Nordisk and Professor Kurt Gothelf’s laboratory at Aarhus University yields novel method to engineer large multi-antibody-like nanostructures using DNA nanotechnology. Assembly of an artificial IgM from a DNA-antibody conjugate and a small 5-way DNA structure. The structure is characterized by Transmission Electron Microscopy (100 x 100 nm). (Image credit: Thorbjørn B. Nielsen and Kurt Gothelf with permission from Angewandte Chemie Int. Ed)
Collaboration between Novo Nordisk and Professor Kurt Gothelf’s laboratory at Aarhus University yields novel method to engineer large multi-antibody-like nanostructures using DNA nanotechnology. Assembly of an artificial IgM from a DNA-antibody conjugate and a small 5-way DNA structure. The structure is characterized by Transmission Electron Microscopy (100 x 100 nm). (Image credit: Thorbjørn B. Nielsen and Kurt Gothelf with permission from Angewandte Chemie Int. Ed)
Over a period of five years, a three-year research scholarship, molded as co-funded Industrial PhDs, was awarded to a total of nine PhD students. The key objective of this strategic collaborative alliance is to additionally develop outstanding research within protein as well as peptide-based drug development at Novo Nordisk (NN) and at Aarhus University (AU).
Now, in a recent publication of a novel efficient technique for linking tiny pieces of proteins bound to short DNA strings to antibodies, a step towards this objective has been undertaken. Researchers at Novo Nordisk and Kurt Gothelf’s research team (Gothelf Lab) developed the novel technique, and Thorbjørn B. Nielsen, the first author of the paper published in Angewandte Chemie Int. Ed. was one among the first PhD students to be enrolled in the aforementioned AU-NN joint industrial PhD program. In addition, the study is being carried out within the framework of the Center for Multifunctional Biomolecular Drug Design, which was initiated the previous year as part of the Novo Nordisk Foundation Challenge Program.
Emulating naturally occurring molecules and components, similar to antibodies can act as a robust tool in analyzing biological mechanisms. In this work, DNA nanotechnology was used to combine DNA structures and protein function, and the former’s nucleic acids (DNA building blocks) were utilized as biological engineering materials instead of the carriers of genetic information existing in living cells. DNA nanostructures provide certain benefits, for instance, the production is scalable and can be developed in the dimensions applicable to scientific experiments with cells and to clinical applications. Complex functionalities are added when proteins are attached to DNA structures, and these functionalities can enable the structures to serve as drugs, prolong the molecules’ lifespan, or direct the structures towards particular molecules.
In this study, the scientists demonstrated the development of a novel technique for connecting a DNA piece to a particular spot on immunoglobulin Gs (IgGs), which are known to be the most prevailing antibodies in the human bloodstream. The connection, or labeling, is guided by a tiny protein piece (peptide), with affinity to a particular spot on the antibody, which is capable of positioning one piece of double-string DNA. Together with half of the DNA double-string, the peptide is then removed easily, leaving behind a single DNA string that is chemically attached to the antibody. This enables connecting the DNA-antibody conjugate to structures with a complementary string of DNA attached.
Scientists have exploited the DNA-conjugates to form an IgM-like nanostructure (pseudo-IgM)—a huge star-like pentameric DNA nanostructure created by arranging five DNA-antibody conjugates.
It is very difficult to link DNA to an antibody in a specific and efficient manner and I am very excited about this work since it offers a solution to exactly that challenge. This is important since it allows us to assemble multiple complex biomolecules in a very well-defined manner just by mixing the components as demonstrated for the pseudo-IgM. The DNA strands are designed to control the self-assembly. The work has only been possible through a wonderful collaboration with Novo Nordisk, where they have contributed with their strong expertise in peptides and the researchers at iNANO including my colleague Professor Jørgen Kjems’ group have contributed with bioconjugation and nanocharacterization. Everything happened in close collaboration in particular by the joint PhD student and first author Thorbjørn Nielsen, who did a fantastic job.
Kurt Vesterager Gothelf, Professor¸ Aarhus University
I feel that Novo Nordisk has only just started the journey into DNA-modified protein drugs with Aarhus University. By combining our expertise in protein and peptide engineering with the unique DNA technologies developed by Kurt Gothelf’s Laboratory, the project team led by senior scientists Emiliano Cló and Anne Louise Bank Kodal has creatively developed a novel molecular scaffold with promising opportunities for therapeutic applications. We hope that the developed technologies can help us understand critical protein-protein interactions of drug targets currently explored at Novo Nordisk. A virtually unexplored scientific field is evolving in front of us, and we cannot predict where it will take us, but opportunities are clearly huge. Needless to say; we are overly excited to collaborate with Aarhus University on DNA-modified proteins in the hunt for new biopharmaceuticals, now and in the future.
Thomas E. Nielsen, Director, Research Chemistry, Novo Nordisk A/S
The Innovation Fund Denmark, Novo Nordisk Foundation (CEMBID), and the Danish National Research Foundation (CDNA and CellPat) financially supported the study. The research was performed by scientists from Interdisciplinary Nanoscience Centre (iNANO), Department of Chemistry and Department of Molecular Biology and Genetics and in association with Novo Nordisk A/S. Professor Kurt Vesterager Gothelf has been in charge of the research group behind the study.