Synopsis
Toshihiro Kamei, Senior Researcher, of the Microsystem Group (Masatake Kanemaru, Group Leader) of the Nanoelectronics Research Institute (Toshimi Wada, General Manger) in the National Institute for Advanced Industrial Science and Technology (AIST; Hiroyuki Yoshikawa, President) has developed a micro fluorescence detector integrating an optical interference filter on an amorphous silicon photo-diode (detection limit: 7 nM of fluorescein, where nM is a concentration of 10-9 Mol/liter).
Toshihiro Kamei, jointly with the Nano-bioanalysis team (Mituru Ishikawa, Group Leader) of the Health Technology Research Center (Tomokuni Kokubu, General Manager) has also succeeded in the separation/detection of DNA fragments at a high-speed (approximately 2 minutes), a high-sensitivity (detection limit of DNA: approximately 250 pg/µL, where 1 pg = 10-9 g) at a high resolution (number of theoretical plates: approximately 70,000) using a hand-held, compact device combined with a microfluidic electrophoresis chip (Figure 1).
The size of the device is comparable to that of home CD/DVD drives, and it can be applied to high-speed analysis of DNA, RNA, proteins, saccharide chains, etc. Thus our device paves the way for high-speed point-of-care bio-analysis, such as for operations in hospitals or emergency medical treatment in ambulances and disaster sites.
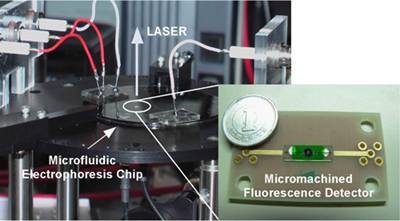
Figure 1. A portable high-speed DNA analysis device incorporating a micro fluorescence detector.
Background to Research Work
With the current progress of Human Genome Project and molecular level understanding of diseases, many things, for example, marker molecules for specific diseases, and the difference between the individual tolerances against diseases and side effects of medicines, caused by the difference between individual genetic information, have been clarified.
However, to apply the current results to operation in hospitals, and emergency treatment in ambulances and disaster sites, high-speed detection and identification of disease markers for immediate understanding of the genomic constitution of patients are necessary. Moreover, the usual medical front also needs diagnosis, dosage, and treatment, which are based on genetic information on patients (tailored medical care). Thus, high-speed, high-resolution, portable bio-analysis devices which can be immediately used at the point-of-care have been desired.
History of Research Work
To meet the need for high-speed and precision, portable bio-analysis devices which are immediately usable at the point-of-care, microfludic biochips enabling high-speed and -precision analysis with a small enough amount of samples to reduce the burden on patients have been developed. However, as the amount of target substances to be detected is very small, even when hand-held microfluidic biochips are used, large-sized high-sensitivity detection systems, such as confocal laser-fluorescence detection systems, must currently be used to measure them (Figure 2).

Figure 2. Microfluidic biochip measuring system with current confocal laser fluorescence detection system. As a large-sized laser and optical detection device are not co-axial, their integration is difficult.

Figure 3. (A) Cross-sectional view of a microfluidic biochip measuring system with the micromachined fluorescence detector we have developed. (B) Top view of the fluorescence detector. he laser and the micromachined fluorescence detector has coaxial configuration while placed in the same side against microfluidic electrophoresis chip. A semiconductor laser is used for excitation.
To exploit potential benefits of microfluidic biochips, the miniaturization and integration of the whole system particulary, the detection system, is essential. To do so, AIST has developed fluorescence detection which use mainstream methods for bio-analysis, and can take over a huge amount of resources for a variety of substances and their examples.
Previously, the researchers proposed and demonstrated in practice an integrated fluorescence detector which can be combined with excitation light sources, and must be a key for the portable bio-analysis device mentioned above. This study was also published in Analytical Chemistry (Vol. 75, pp 5300-5305) in 2003. In this work, the researchers have succeeded in the improvement of the limit of detection by integrating and patterning an optical filter on a amorphous silicon photodiode with semiconductor microfabrication (Figure 3).
Support
This research work has been carried out by the support of the “Research and development of high-sensitivity integrated fluorescence detection modules for the point-of-care ultra-parallel biochips” project (in FY years of 2005-2007) of the New Energy and Industrial Technology Development Organization. Furthermore, the amorphous silicon photodiode was prepared by Fuji Advanced Technology Ltd.
Details of Research Work
Amorphous silicon, known as the material of thin film solar cells and thin film transistors which drive liquid crystal displays, has the following features which are almost ideal as fluorescence detectors for bioanalysis.
- Fluorescence bands of many fluorescent dyes are located in the visible light region from 500 to 600 nm. In this region, the absorption coefficient of amorphous silicon is over one order larger than that of crystalline silicon (Figure 4).
- Amorphous silicon enables low-noise measurements even at room temperature, rendering it to indicating that it needs no cooling systems, and is advantageous to its miniaturization.
- Device-grade- amorphous silicon can be made at low temperatures such as 200°C, allowing for a use of inexpensive substrates such as glass and plastics. It is also suited with easy cost reduction and mass production.

Figure 4. Relationship between fluorescence spectra of dyes used for bio-analysis and absorption coefficient of crystalline or amorphous silicon (EtBr, TOTO, FAM, and EGFP are the name of fluorescent dyes)
Geometrical Structures Are Important For Semiconductor Devices
As shown in Figure 3 (A), the significant feature of the micromachined fluorescence detector we have developed in this work is a coaxial configuration of excitation light source and the fluorescence detector that are placed on the same side of a microfluidic electrophoresis chip. As shown in Figure 3 (B), this design exploits optical transparency of a glass substrate to combine an pinhole formed in the center of amorphous silicon photodiode and optical interference filter with vertical laser excitation through the detector glass substrate. Such a configuration makes possible to fabricate a compact module combining an excitation light source and fluorescence detector, reducing efforts significantly for optical axis alignment.
High-Speed, High-Sensitivity, And High-Separation Efficiency Of Dna Fragments Using A Micromachined Fluorescence Detector
The researchers have fabricated a micromachined fluorescence detector shown in Figure 3, in which a SiO2/Ta2O5 thick optical interference filter is integrated and patterned on an amorphous silicon photodiode.
Samples labeled with fluorescence dyes flowing in a microchannel are irradiated by a semiconductor laser, and then fluorescence emitted from them is collected and collimated with a microlens to detect purely the fluorescence component transmitted through the optical interference filter using an amorphous silicon diode.

Figure 5. Dependence of photocurrent and S/N ratio of our micromachined fluorescence detector on fluorescein concentration

Figure 6. DNA fragments with eleven kinds of lengths are separated on a microfluidic electrophoresis chip, and are detected by a micromachined fluorescence detector. Completely raw data; DNA concentration: 100 ng/µL
The performance of this micromachined fluorescence detector was evaluated in terms of fluorescence of a representative fluorescence dye, fluorescein. The limit of detection is determined to be7 nM of fluorescein concentration, as shown in Figure 5, which achieves the commercial level.
Moreover, the researchers have succeeded in the electrophoretic separation and detection of DNA fragments with 11 kinds of lengths by the microfluidic biochip combined with the micromachined fluorescence detector (DNA of a bacteriophage, ÖX174, was digested using a restriction enzyme, Hae III) (Figure 6). The device, including a fluorescence detector, is hand-held, enabling the electrophoretic separation at a high-speed (approximately 2 minutes), high-sensitivity (limit of detection of DNA: approximately 250 pg/µL (1 pg = 10-9 g)), and high-resolution (number of theoretical plates: approximately 70,000).
Future Prospects
The size of the device is comparable to those of home CD/DVD drives, and can be applied to a high-speed analysis for DNA, RNA, proteins, succharide chains, etc. Thus, the device may constitute fundamental platform for point-of-care high-speed bio-analysis, such as for operations in hospitals or for emergency medical treatment in ambulances and disaster sites. The immediate aim is the improvement of the limit of detection down to 1-2 nM of florescein concentrations, enabling more advanced analyses, e.g., DNA sequencing.
In the future, when blue-green vertical cavity surface emitting laser (VCSEL) is realized, micromachined fluorescence detector can be monolithically integrated on the VCSEL, because amorphous silicon semiconductors can be fabricated at 200C, enabling the realization of mass-productive heavily multiplexed fluorescence detection devices. Such devices will play an important role to realize a drastic improvement of the throughput of important bio-analyses such as DNA sequencing.
|