Apr 7 2021
Although DNA sequencing has now turned more common, very few understand how difficult it is to extract even a single molecule of DNA from a biological sample.
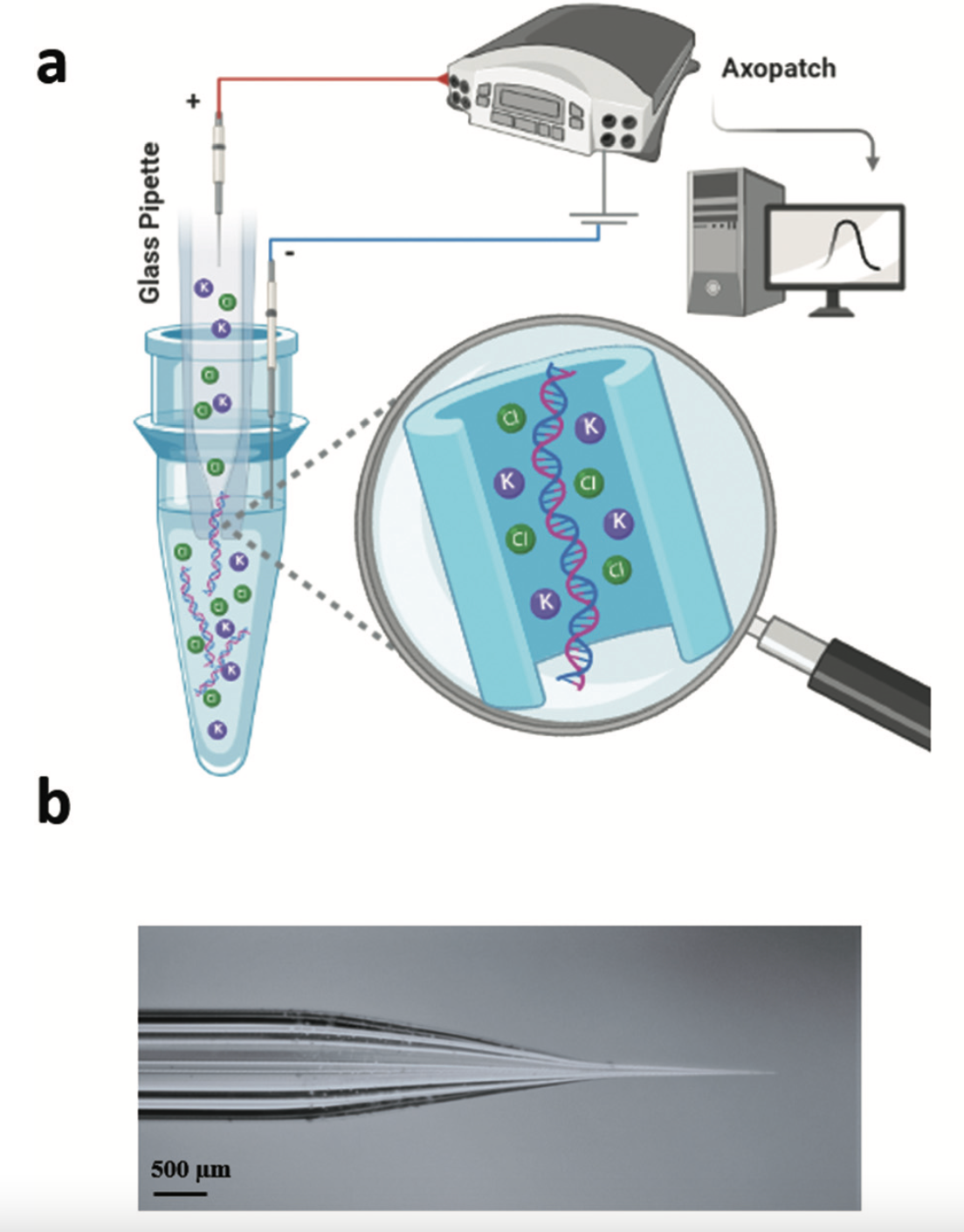 An illustration showing (a) how a glass tube with a tiny opening and a positive electrode, when inserted into a liquid sample and stimulated with electricity, collects cell-free DNA floating in the sample; (b) a photograph of the borosilicate glass nanopore. Image Credit: Freedman et al., 2021.
An illustration showing (a) how a glass tube with a tiny opening and a positive electrode, when inserted into a liquid sample and stimulated with electricity, collects cell-free DNA floating in the sample; (b) a photograph of the borosilicate glass nanopore. Image Credit: Freedman et al., 2021.
Led by UC Riverside, a new study is enabling easy detection and capture of DNA from fluid samples like blood with a tiny glass tube and electric current. The technique has been explained in the Nanoscale journal and can also enhance cancer diagnosis in the future.
DNA is a double-stranded, electrically charged molecule including all the information required by an organism to develop and organize the building blocks of life. It is tightly folded inside the cell nucleus. It is impractical and time-consuming to extract the DNA from a single cell for several scientific and medical purposes.
Luckily, during natural cell death, the membranes of the cells burst and release the contents, which include DNA. This implies that a blood sample, for instance, includes several strands of free-floating DNA that should be theoretically simple to identify and extract in quantity.
But most of the cell-free DNA is destroyed by scavenger cells known as macrophages, which clean up cellular waste. This leaves DNA at low concentrations in the blood.
A majority of the techniques to capture cell-free DNA necessitate high cost techniques that initially concentrate the molecules before employing fluorescent dyes to help visualize the DNA.
Kevin Freedman, the corresponding author of the study and an assistant professor of bioengineering at UC Riverside’s Marlan and Rosemary Bourns College of Engineering, headed an effort to enhance the detection and capture of DNA at lower concentrations.
The method involved using an electric charge to guide a DNA sample directly into a glass tube that features a small opening known as a nanopore.
Nanopore sensing has become a cost-effective, rapid, and reliable diagnostic tool in various clinical and medical applications.
We know that if you apply voltage across a cell membrane, ions will move through pores in the cell membrane. DNA also travels with the electric field, and we can use it to move the DNA.
Kevin Freedman, Assistant Professor of Bioengineering, Marlan and Rosemary Bourns College of Engineering, UC Riverside
A positive electrode was placed inside a glass tube featuring an opening, or pore, with a width of 20 nm—somewhat wider than a DNA molecule but too small to allow cells. An electrical potential was applied to the nanopore, which was immersed into a vial that contained a negative electrode and a DNA sample.
The cell-free DNA entered the pore and blocked it. By using the change in electrical current when the DNA moved through the pore, the researchers could detect it.
It’s like trying to pull spaghetti through a needle. To go through the pore it has to be almost perfectly linear.
Kevin Freedman, Assistant Professor of Bioengineering, Marlan and Rosemary Bourns College of Engineering, UC Riverside
The distance at which the pore was held from the liquid surface governs the quantity of DNA it picked up. If the distance is closer, then more DNA is captured.
Amazingly, we found that DNA accumulates at the liquid-air interfaces. If there is a cooling layer, the DNA will try to go to the cooler location. We hope the same is true for a blood sample, so the same mechanism can be used to concentrate DNA near the surface. Not only is this beneficial, but this nanopore-sensing strategy demonstrated a higher signal-to-noise ratio near the surface as well. It is really a win-win situation.
Kevin Freedman, Assistant Professor of Bioengineering, Marlan and Rosemary Bourns College of Engineering, UC Riverside
The researchers believe that with some fine-tuning, their purely electric technique could enable the diagnosis of certain types of cancer using a single blood sample. Apart from DNA, the growth of tumors releases vesicles into the bloodstream.
These mini lipid-based droplets can be considered as mini-cells similar to the original cancer cells and could even be detected through nanopore sensing.
When all the exclusive features of this purely electrical technique are taken into account, the nanopore-sensing system has the capability to serve as a point-of-care diagnostic test evaluation in the future.
Nasim Farajpour, Lauren S. Lastra, and Vinay Sharma, all from UC Riverside, joined Freedman in the study.
Journal Reference:
Farajpour, N., et al. (2021) Measuring trapped DNA at the liquid-air interface for enhanced single molecule sensing. Nanoscale. doi.org/10.1039/D0NR07759C.