A recent article published in the journal Nanomaterials describes a novel eco-friendly material synthesis technique that can be used to manufacture lithium-ion batteries for clean energy production.

Study: Eco-Friendly Water-Processable Polyimide Binders with High Adhesion to Silicon Anodes for Lithium-Ion Batteries. Image Credit: petrmalinak/Shutterstock.com
Researchers used water as the solvent in a straightforward one-step approach to manufacturing an environmentally friendly, water-soluble polyimide precursor salt that serves as a binder for silicon anodes. This can then be used to manufacture lithium-ion batteries with a high discharge capacity and good cycling performance.
In recent years, electric vehicles (EVs) and energy storage systems (ESSs) have been produced rapidly as alternatives to fossil fuel consumption to minimize greenhouse gas emissions. These systems use lithium-ion batteries (LIBs), which offer several desirable characteristics, including a high working voltage, a high energy density, and a low self-discharge rate. However, for the commercialization of EVs and ESSs to be successful, the energy density of LIBs must be increased.
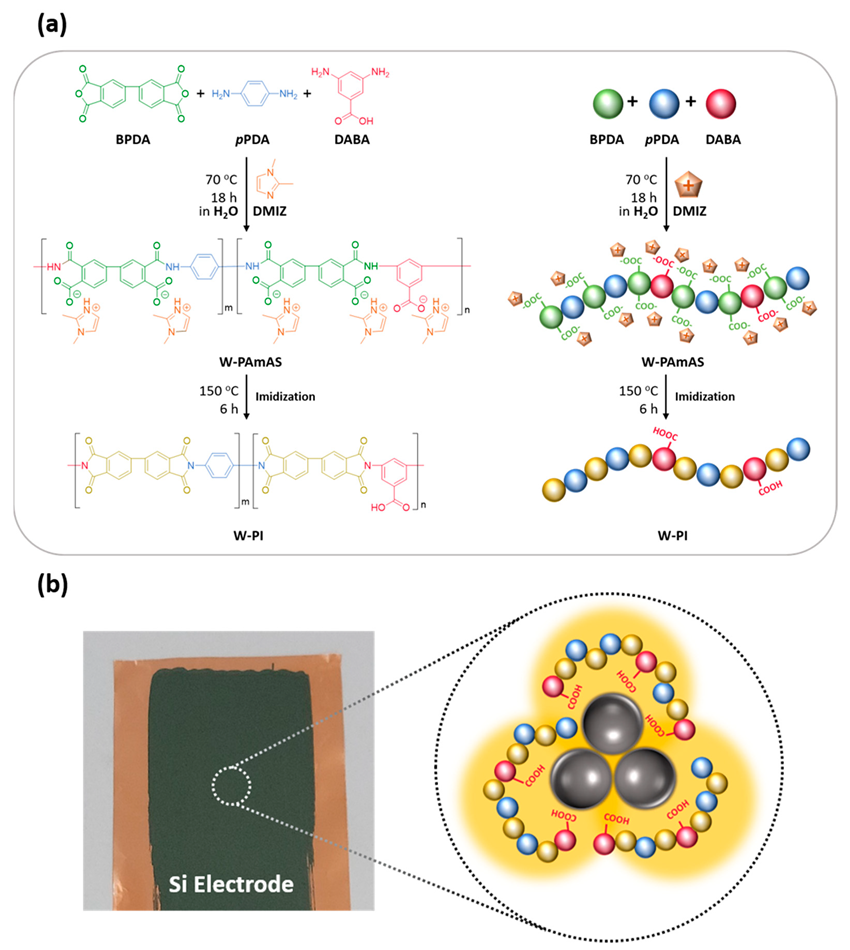
(a) Synthesis scheme of W-PAmAS-30 and preparation of W-PI-30. (b) Photograph of the Si electrode on Cu foil fabricated using W-PI-30 as a binder after thermal treatment at 150 °C for 6 h and schematic illustration of chemical interactions between SiNPs and the W-PI-30 binder. Image Credit: So, Y. et al.
Applications and Limitations of Silicon
A viable solution to the LIBs’ energy density problem is to make use of silicon-based anodes. It is abundantly present and has an extraordinarily high theoretical discharge capacity paired with a low operating voltage compared to ordinary graphite anodes.
Despite these advantages, there are still some issues with the production of silicon-based electrodes. For example, these electrodes can undergo a 300% morphological shift during lithiation.
|Frequent lithiation and delithiation processes can place significant mechanical strain on the silicon-electrodes, resulting in fracture of the silicon-active elements and corrosion of the copper current collector. This reduces the contact area and an elevation in resistance, which significantly impairs the silicon electrode's cycling performance.
An Eco-Friendly Technique for Synthesis of Polyimides (PIs)
Previously, deterioration of silicon anodes was minimized by employing a polymeric binder modification technique utilizing polyimides (PIs) because of their excellent mechanical capabilities, chemical inertness, heat resistance, and impermeability. However, solvents with high polarities, such as dimethylacetamide and N-methyl2-pyrrolidone, are necessary for the polymerization process. Due to the harmful environmental impact and high cost of these organic solvents, their usage in the industrial sector has been greatly limited in recent times.
In this work, the researchers created a water-based synthesis process for polyimides that is both eco-friendly and cost-effective. By adding an organic base to water, an aqueous poly (amic acid) salt can be generated in a single step. Additionally, as compared to conventional organic-based PAmA, water-soluble PAmAS may lower the imidization temperature.
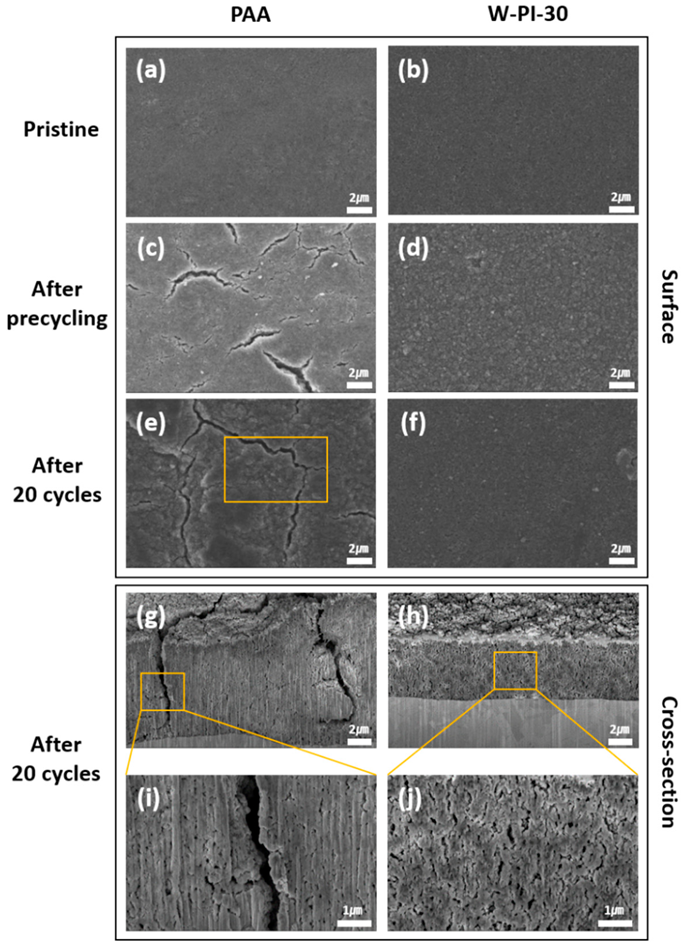
FE-SEM images of Si electrodes with PAA and W-PI-30 binders. (a–f) Surface morphology of Si electrodes before cycling, after precycling, and after 20 cycles ((a,c,e) PAA and (b,d,f) W-PI-30). (g–j) Cross-sectional images of Si electrodes with PAA and W-PI-30 binders after 20 cycles. Image Credit: So, Y. et al.
Research Methods
Firstly, the researchers used 100mL deionized water flasks mixed with 25mmoL DMIZ and 10mmoL diamine to make a water-soluble poly salt (W-PAmAS) in an N2 environment. The solution was heated and agitated for 18 hours at 70 degrees Celsius.
After polymerization, a sticky mixture with an orange-brown appearance was formed, with an intrinsic viscosity of 0.46 dL g-1 in the W-PAmAS-30 waterborne solution. After that, a copper foil was covered with an aqueous silicon electrode solution including SiNPs, conductors, and freshly prepared W-PAmAS.
A roller was used to modify the thickness of the silicon electrodes, which were then cut into discs with a width of 12 mm and treated at 150 degrees Celsius for six hours in a vacuum. The electrolytic characteristics of the silicon electrodes were tested using CR2032 lithium metal cells.
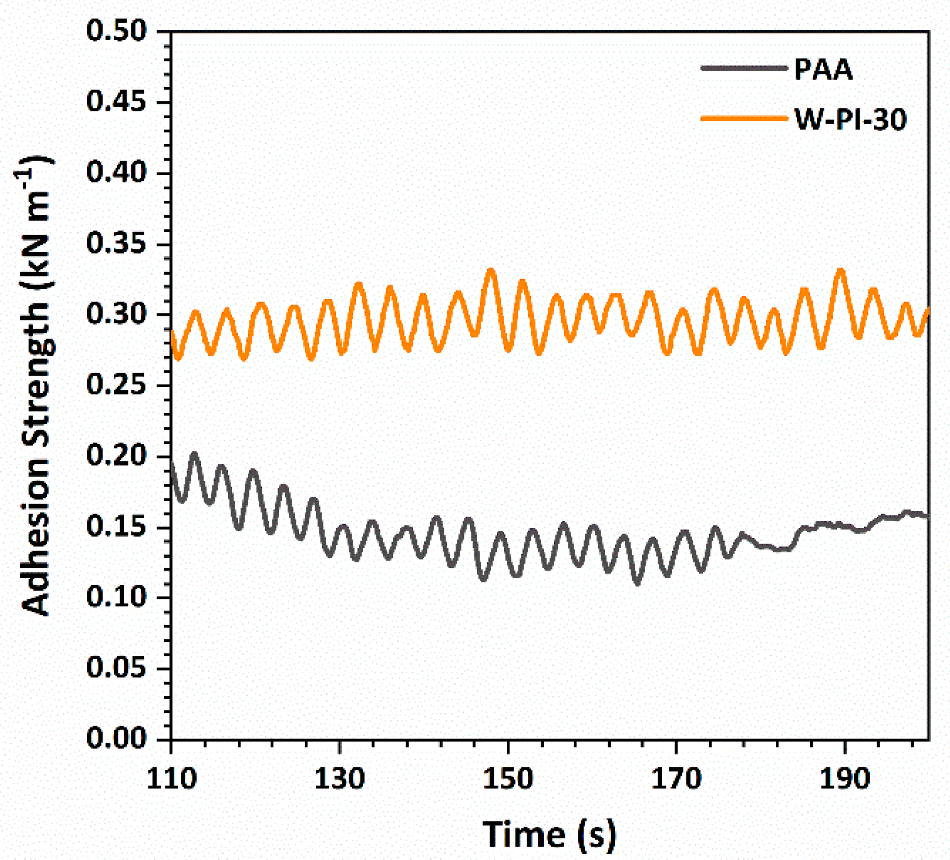
Adhesion strength of Si electrodes with PAA and W−PI−30 binders measured using SAICAS. Image Credit: So, Y. et al.
Significance of Water-Based Polyimide Synthesis Technique
In this work, a simple and cost-effective approach generated an eco-friendly water-soluble PI binder (W-PAmAS) for silicon anodes. The strong covalent connections formed when polyimide binders were added to silicon electrodes considerably minimized volume variations in the Si-electrodes throughout the alloying and dealloying process. Furthermore, since an imidization level of >90% was attained when W-PAmAS was heated, this low-temperature technique reported can help deter undesired corrosion of the copper current collector during electrode production for LIBs.
Batteries with silicon-electrodes and a water-based PI binder demonstrated excellent catalytic performance, retaining 91.3% of their initial discharge capacity after 200 cycles and performing well even at high current densities. Additionally, the adhesive strength of the water-based PI binder was almost twice that of the standard PAA binder. These incredible results are due to the fact that better covalent bonding inhibits the morphological alterations of Si-electrodes.
To conclude, it can be said that this novel technique offers a great deal of promise for the synthesis of eco-friendly water-soluble binders as an adhesion material for Si-anodes with adequate electrochemical properties for lithium-ion batteries.
Continue reading: Graphene Oxide Nanoparticles in Biodegradable Lubricant Synthesis.
Reference
So, Y. et al. (2021). Eco-Friendly Water-Processable Polyimide Binders with High Adhesion to Silicon Anodes for Lithium-Ion Batteries. Nanomaterials, 11(12). Available at: https://www.mdpi.com/2079-4991/11/12/3164
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.