A recent study published in the journal Nanomaterials explores the development of a carbon nanofoam interlayer for use in lithium-sulfur batteries.
Study: Structural and Surfacial Modification of Carbon Nanofoam as an Interlayer for Electrochemically Stable Lithium-Sulfur Cells. Image Credit: Smile Fight/Shutterstock.com
This research enables the sulfur cathode to achieve excellent electrochemical productivity, effectiveness, and robustness. The carbon-nanofoam interlayer is permeable and helical, accelerating charge transport while retarding polysulfide propagation. The enhanced cell displays an electrolytic efficiency of over 80% and 200-cycle durability.
Between the oxide cathode and carbon anode of modern Li-ion fuel cells, an intervention reaction mechanism is used to reversibly discharge and absorb lithium ions. Ceramics' robust crystal lattice ensures high cycle durability, which is critical for the continued growth of the rechargeable battery industry. However, the charge-storage capabilities of Li-ion cell cathode materials have almost achieved their predicted maximums in advanced lithium-ion systems.
Importance of Lithium-Sulfur Batteries
To address the ever-increasing need for energy storage worldwide, the production of next-generation lithium-ion batteries requires innovative cathode materials with increased charge storage space and long-term ionic conductivity.
Among the recently produced rechargeable batteries, lithium-sulfur fuel cells employ sulfur as the cathode material to create a higher electron-storage capability during a transformation battery chemical process, allowing the system to produce a high energy density and achieve excellent electrical and chemical performance.
Additionally, the sulfur used is low-cost, harmless, and plentiful. As a result of their electrolytic and material characteristics, lithium-sulfur cells have significant applications in different fields.
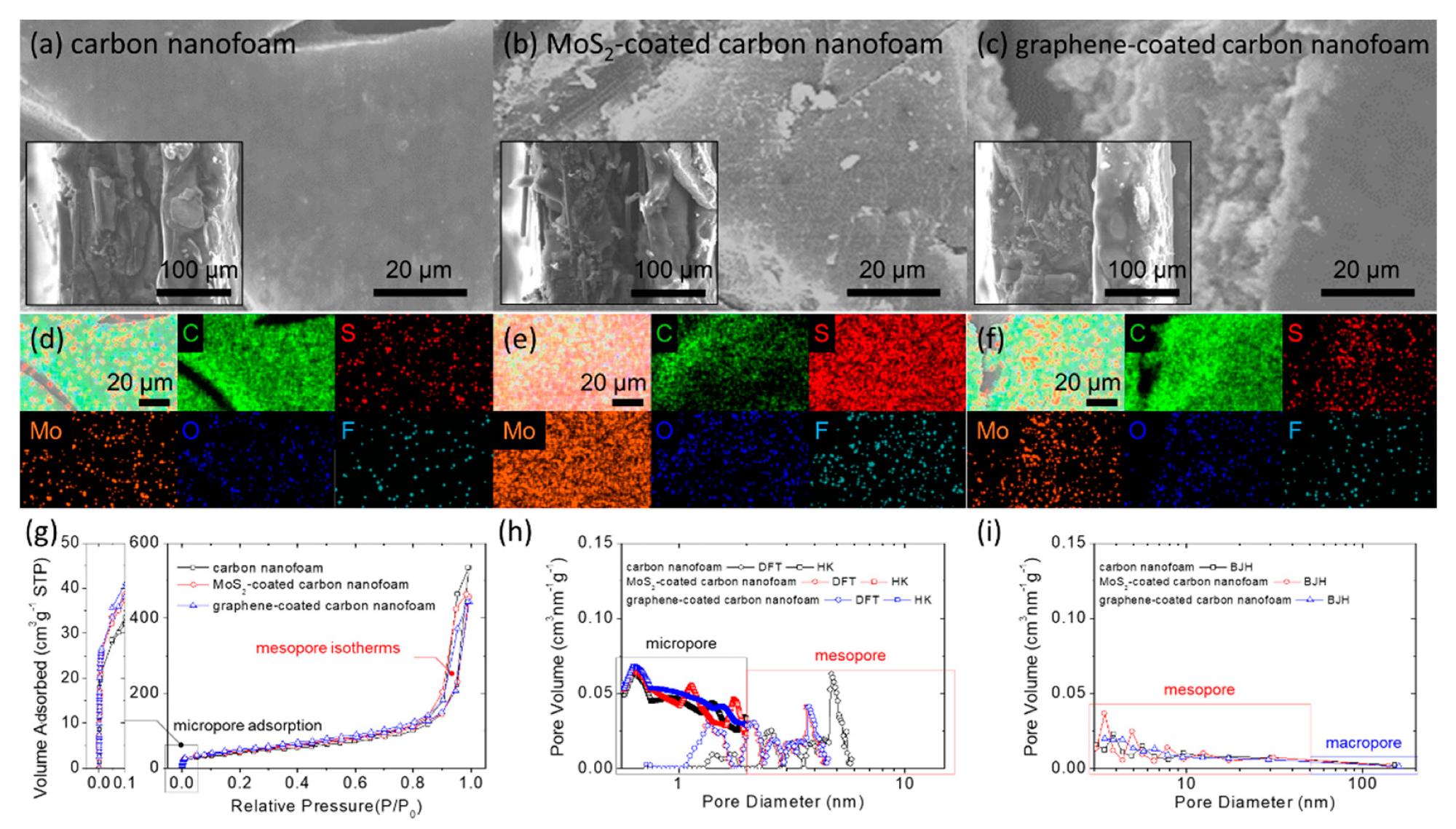
Material characterization: microstructural inspection of the (a) carbon nanofoam, (b) MoS2-coated carbon nanofoam, and (c) graphene-coated carbon nanofoam by field-emission scanning electron microscopy (insets are the cross-sectional inspection); elemental mapping results of the (d) carbon nanofoam, (e) MoS2-coated carbon nanofoam, and (f) graphene-coated carbon nanofoam by energy-dispersive X-ray spectroscopy; porosity analysis with (g) nitrogen adsorption/desorption isotherms, (h) pore size distribution calculated with density functional theory (DFT) and Horvath–Kawazoe (HK) theory, and (i) Barrett-Joyner-Halenda (BJH) pore size distribution. © Quay, Y.-J., & Chung, S.-H. (2021)
Challenges in Fabrication of Lithium-Sulfur batteries
The rapid advancement of battery cells has encountered inherent material difficulties due to the poor charge transport properties of solid-state sulfur as well as the creation and dispersion of fluid polysulfides. External electrolytic constraints in fabricating high-energy-density electrodes with a sufficient active layer in an electrolyte unit have also hampered lithium-sulfur battery applications.
At the complete charge and discharge phases of the Li-S battery, the active substance transforms into solid-state materials such as sulfur and alkali sulfides. Both solid-state materials are excellent insulators, which restrict the reactive substance's electrolytic usage and cyclability during redox processes.
At the secondary phases of discharging and charging the cell, the solid-state active constituents transform into fluid lithium polysulfides, which rapidly disperse out of the sulfur cathode area and spread wildly across the battery. Due to the permanent polysulfide dispersion, the active material rapidly degrades and the electrodes and electrolyte deteriorate, resulting in low cyclability and inconsistent discharge and charge performance.
Unfortunately, this cell structure limits the production and analysis of high-energy-density lithium-sulfur cells, which need high-loading sulfur cathodes to reach maximum sulfur electrochemical efficiency.
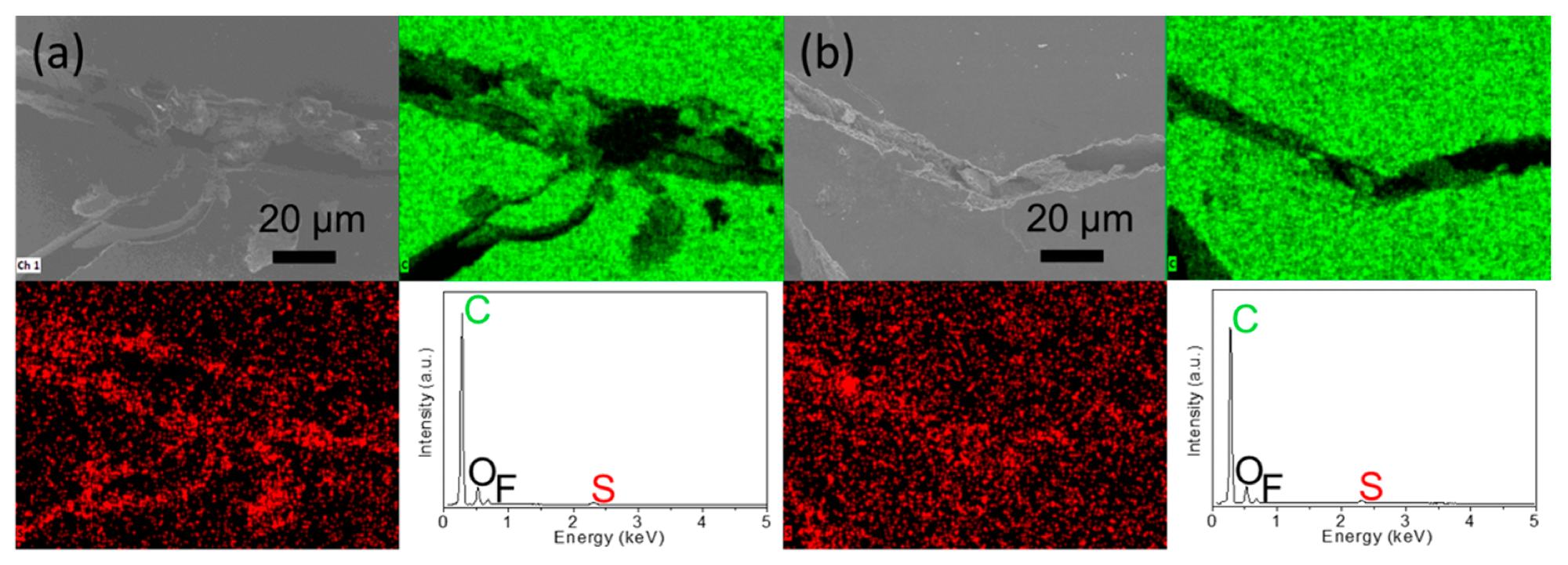
Electrochemical analysis: microstructural inspection and elemental mapping results of the inserted carbon paper for the polysulfide diffusion analysis: (a) the side facing cathode and (b) the side facing anode. © Quay, Y.-J., & Chung, S.-H. (2021)
A Novel Technique for Fabrication of Li-S Batteries
In this study, the researchers modified the lithium-sulfur battery by interlaying carbon nanofoam between the sulfur electrode and the divider. The interlayer's highly conductive structure enhances the cathode conductance, resulting in increased sulfur electrolytic efficiency.
During cycling, the layer provides a helical and permeable matrix that inhibits the movement of liquid-state sulfides. Stabilization of the trapped sulfides occurs inside the carbon interlayer and electrode area, ensuring that the active material maintains a high chemical efficiency level. As a result, electrolytic conversion and cycle stability are improved.
Important Research Findings
The researchers discovered that a lithium-sulfur battery with a carbon-nanofoam interlayer allowed the electrode to maintain the highest sulfur content and sulfur loading while achieving a current-storage capability of 1087 mAh g-1 at a low electrolyte-to-sulfur proportion.
The high-loading sulfur cathode displayed a better aquifer potential of 8.7 mAh cm-2 and an exceptional power density of 18.7 mWh cm-2, both of which are similar to contemporary lithium-ion batteries. According to the comparative study of the cell structure and material attributes, the activated carbon nanofoam interlayers also enhanced the general electrolytic properties of lithium-sulfur batteries.
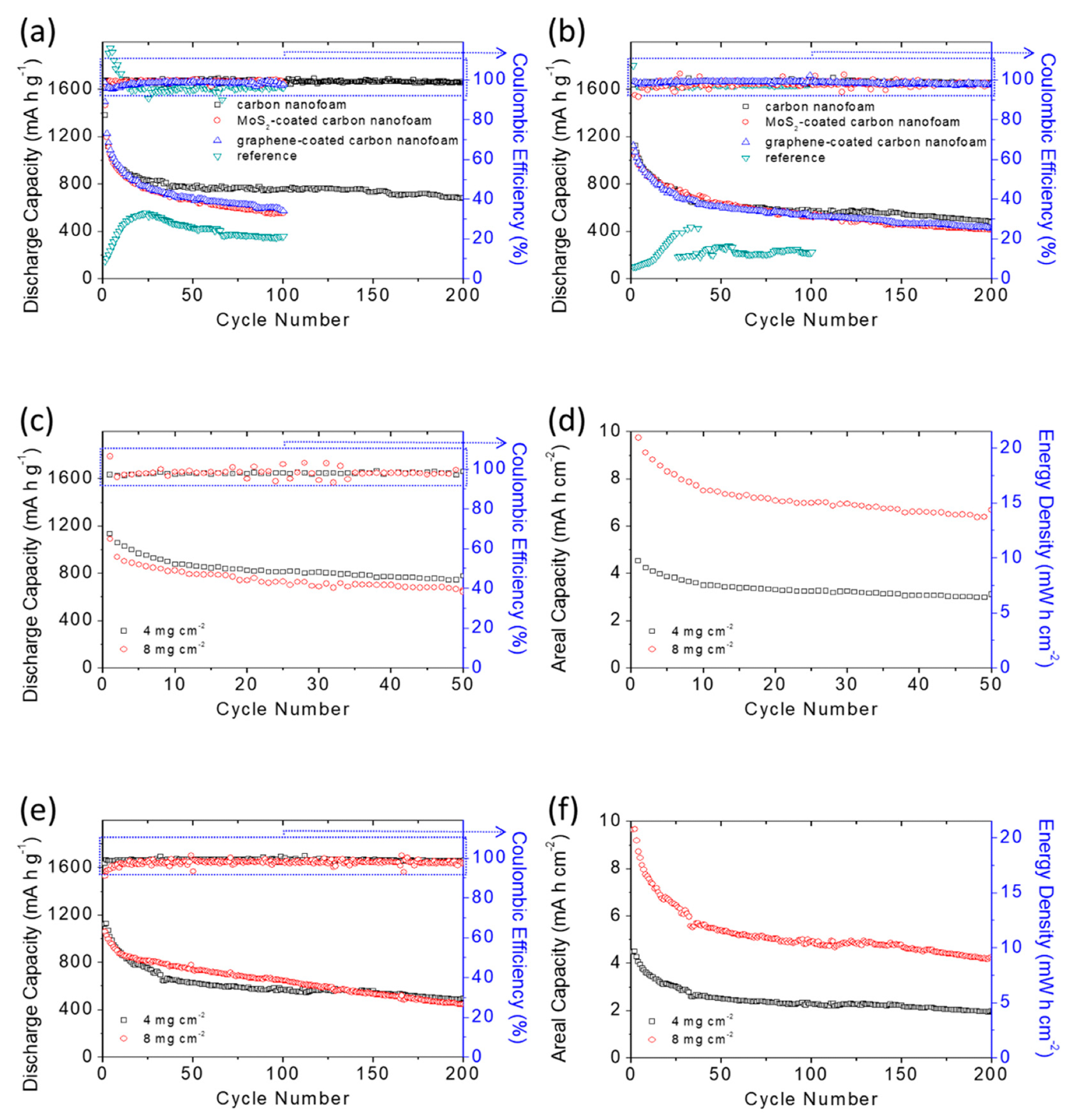
Battery performance analysis: cyclability of the sulfur cathodes with various carbon-nanofoam interlayers at (a) the C/20 rate and (b) the C/10 rate; battery performance and areal capacity and energy density analysis of the high-loading sulfur cathodes with the carbon-nanofoam interlayer at (c,d) the C/20 rate and (e,f) the C/10 rate. © Quay, Y.-J., & Chung, S.-H. (2021)
Future Perspective
The carbon-nanofoam interlayers provide significant performance increases, exceeding the values required to power electric cars and serve as a replacement for traditional lithium-ion battery cathodes. Furthermore, a comparison of the Li-S using a carbon-nanofoam interlayer with other advanced sulfur-loading batteries reveals that the carbon-nanofoam interlayer can ensure a remarkable balance in power efficiency and electrochemical properties.
Continue reading: Sila Nanotechnologies: A Next-Gen Lithium-Ion Battery Solution.
Reference
Quay, Y.-J., & Chung, S.-H. (2021). Structural and Surfacial Modification of Carbon Nanofoam as an Interlayer for Electrochemically Stable Lithium-Sulfur Cells. Nanomaterials, 11(12), 3342. Available at: https://www.mdpi.com/2079-4991/11/12/3342
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.