The development of core/shell Ag/SnO2 nanowires as a novel photocatalytic material for the fast breakdown of organic molecules by light waves is the subject of a recent study published in the journal Catalysts.

Study: Core/Shell Ag/SnO2 Nanowires for Visible Light Photocatalysis. Image Credit: agsandrew/Shutterstock.com
After being coated with a SnO2 shell, silver nanowires alter their optical characteristics and may be stimulated by visible light. This property of Ag/SnO2NWs can be used to develop effective and quick photocatalytic degradation systems to eliminate organic contaminants under natural sunlight without the use of any additional radiation devices.
SnO2: Applications & Limitations
Due to increased environmental contamination and rapid industrial expansion, there has been a surge of attention to developing effective, quick, and broadly applicable photocatalytic systems based on nanoscale materials in the last decade. Semiconductor materials, particularly metal oxides, have been widely explored as photocatalysts due to their unique physio-chemical characteristics.
As a semi-conductive metal oxide with excellent biochemical, physical, and structural stability, as well as excellent performance in the degradation of organic pollutants, SnO2 is among the most promising materials to be discovered in recent years. However, the inability to use visible light for real-world applications is a significant obstacle to the widespread use of tin oxide (SnO2).
Due to the high bandgap and the very rapid coupling of photoelectrons and holes, the effective deployment of SnO2 for industrial applications is severely restricted.
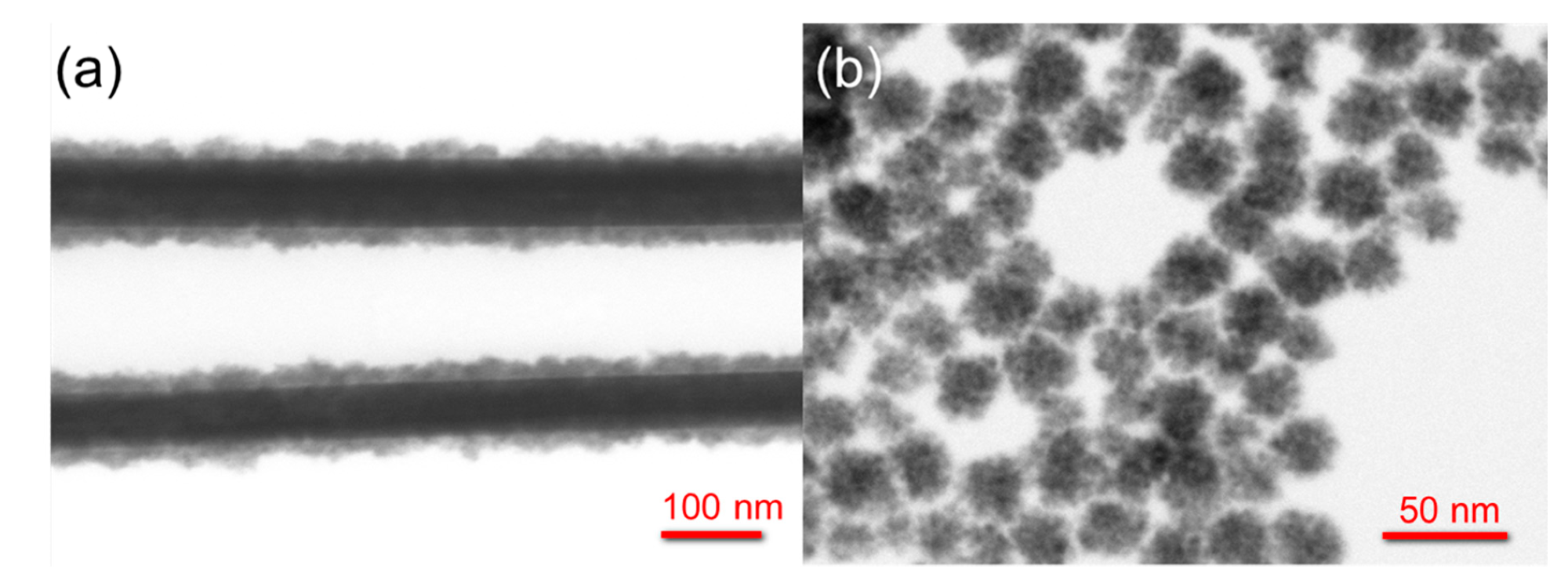
STEM images of (a) core/shell Ag/SnO2NWs and (b) SnO2NPs. © Baranowska-Korczyc, A. et al. (2021)
Ag Nanostructures for SnO2 Applications
Different metallic nanostructures have been used to build a nanocomposite with semiconductors to produce effective carrier segregation under solar light illumination to address these constraints. Using this approach, an electrochemically active biofilm may be made to produce an Ag-SnO2 hybrid that can be employed as a visual light-driven photocatalyst to break down various organic molecules.
The one-pot hydrothermal process can be used to make an Ag/SnO2 hybrid that is very efficient in photodegrading phenol. Under light waves, Ag-doped SnO2 nanostructures altered with turmeric are an effective photocatalyst for the breakdown of rhodamine B.
Ag-SnO2 nanomaterials can not only be employed as photocatalysts, but they also have excellent antioxidant and antimicrobial capabilities.
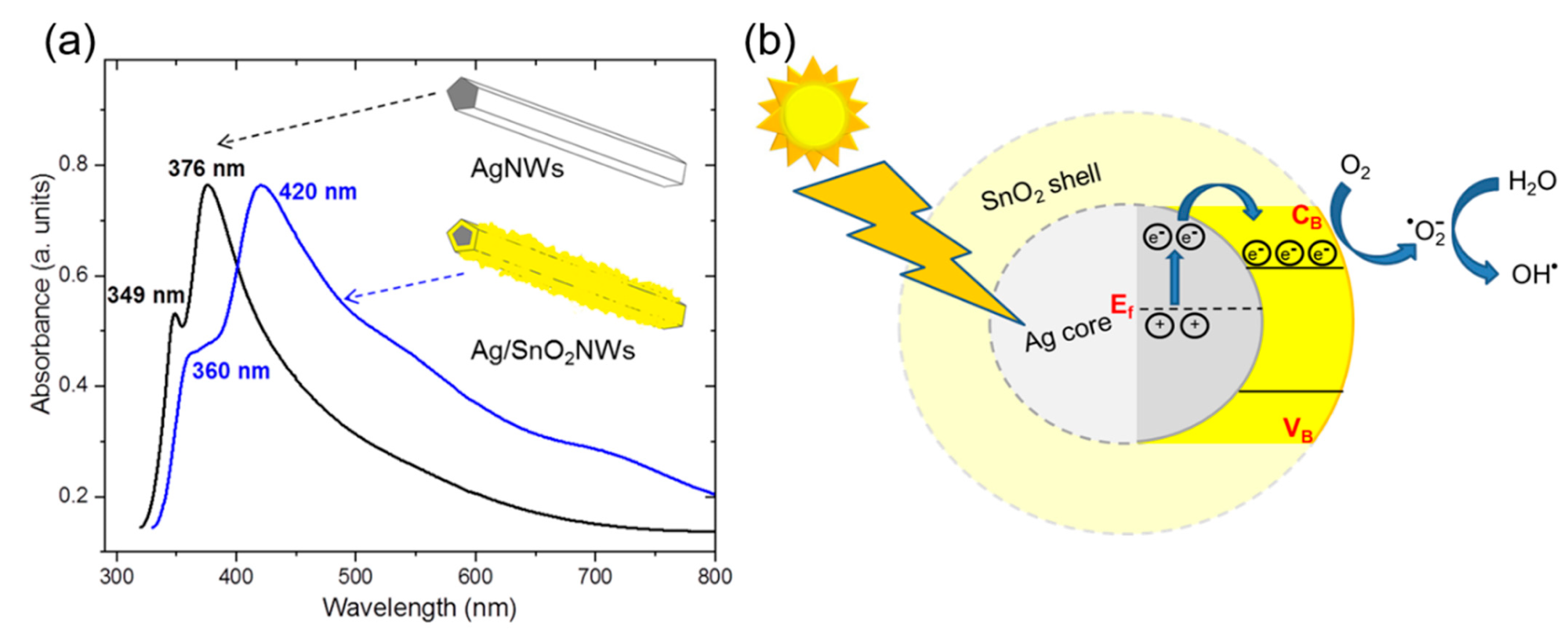
(a) The absorbance spectra of AgNWs and the core/shell Ag/SnO2NWs. (b) Schematic illustration of the photocatalytic mechanism of Ag/SnO2NWs under visible light irradiation. © Baranowska-Korczyc, A. et al. (2021)
Importance of Silver Nanowires
Although silver nanoparticles of different dimensions can be utilized to make Ag/SnO2 compounds for catalytic applications, Ag nanowires (AgNWs) have never been exploited for this purpose, despite their numerous benefits.
Strong transparency, good electromagnetic capabilities, excellent electrical efficiency, mechanical stability, nanometric scale, and one-dimensional (1D) shape are all characteristics of AgNWs. Furthermore, in contrast to other silver nanoparticles, the AgNWs' easy separation by filtering or centrifugation allows for simple manufacturing and boosts their usability.
Both nanomaterials' advantageous properties can be combined in the core/shell Ag/SnO2NWs heterostructure to achieve exceptional catalytic capabilities.
In this study, the researchers offer a novel photocatalytic system based on AgNWs covered with a SnO2 shell. To explore the photocatalytic degradation mechanism using a new 1-D Ag/SnO2NWs catalyst, rhodamine B and malachite green were chosen as sample organic dyes and hazardous dyes that are both prevalent in the atmosphere. The deterioration was evaluated by looking at the dye solution's UV absorbance over time.
Research Findings and Conclusion
Under solar light illumination, silver nanowires with a SnO2 shell can be used as a novel and effective photocatalyst. The quick decomposition process is the result of the combination of benefits of both elements in the core/shell Ag/SnO2NWs hybrid.
The SPR absorption range of AgNWs after treatment with SnO2 moves towards the visible range, allowing photons of this frequency to excite electrons in the nanowires.
SnO2's photocatalytic efficiency is greatly increased even without UV illumination. The electrical pulses from the metal core are transported to the transition metal shell and grabbed by oxygen molecules, resulting in the generation of radical species that are necessary for organic compound decomposition.
The core/shell metal/semiconductor configuration, which leads to carrier isolation and avoids mixing photoinduced electron-hole pairs, is credited with the Ag/SnO2NWs system's remarkable photocatalytic performance. For photocatalytic applications, the ease with which an Ag/SnO2NWs hybrid may be processed using simple separation methods like filtering or centrifugation is highly advantageous.
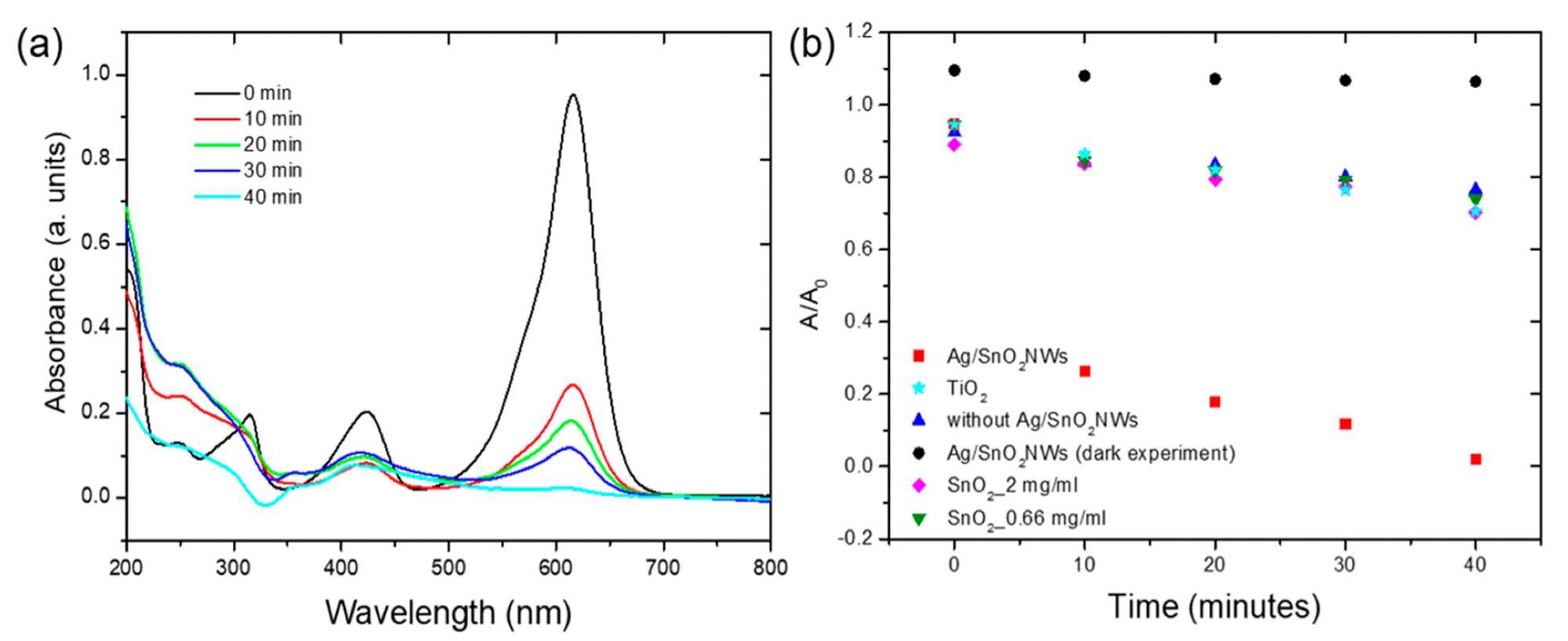
(a) The absorbance spectra of malachite green with Ag/SnO2NWs photocatalyst presence under 450 nm light irradiation. (b) The degradation of malachite green with 2 mg/mL of Ag/SnO2NWs catalyst (■), with no photocatalyst (▲), with 0.66 mg/mL of SnO2NPs (▼), 2 mg/mL of SnO2NPs (◆), and with 2 mg/mL TiO2 catalyst (★) under 450 nm light irradiation and Ag/SnO2NWs without irradiation (dark experiment, ●).© Baranowska-Korczyc, A. et al. (2021)
Future Perspective
According to the results, the core/shell of Ag/SnO2NWs is a highly promising material with great environmental sustainability for constructing future photo electrocatalytic systems for successful remediation operations of diverse organic compounds under direct solar radiation.
Continue reading: Could Nanoparticles Create Sustainable Lighting?
Reference
Baranowska-Korczyc, A. et al. (2021) Core/Shell Ag/SnO2 Nanowires for Visible Light Photocatalysis. Catalysts, 12(01), 30. Available at: https://www.mdpi.com/2073-4344/12/1/30
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.