Recently, an article was published in the journal Catalysts, which discusses how Platinum-Gold (PtAu) nanoparticles that have been placed on reduced graphene oxide (rGO) have a considerable catalyzing rate for the evolution of hydrogen in H2SO4 solution.

Study: PtAu Nanoparticles Supported by Reduced Graphene Oxide as a Highly Active Catalyst for Hydrogen Evolution. Image Credit: petrmalinak/Shutterstock.com
Importance of Hydrogen as Fuel
The world desperately needs a viable replacement for fossil fuels, both economically and environmentally.
As hydrogen gas is a clean and cheap source of energy, thus, making it a good fuel, it can be created by decomposition of water into hydrogen and oxygen via the evolving of hydrogen gas.
Since it is so widely applicable, the hydrogen evolution reaction (HER) has been a thoroughly investigated reaction in chemistry owing because of its broad significance from both an academic and industrial standpoint.
These activities are necessary for a variety of operations, such as the production of electricity in hydrogen fuel cells, the production of H2 via water electrolysis, and the conversion of CO2 into useable fuels and chemicals, among others.
A good catalyst for hydrogen evolution reaction (HER) is required for an optimum and inexpensive hydrogen generation process.
Catalysts should be inexpensive while also exhibiting high efficiency, durability, and longevity. Generally, it is acknowledged that metals in the platinum group, with Pt at top of the list, are the greatest electrocatalysts for the hydrogen evolution process, but that they are very costly and difficult to get as electrode materials.
In this study, the researchers explored suitable catalysts for the reaction, which are also inexpensive and readily available.
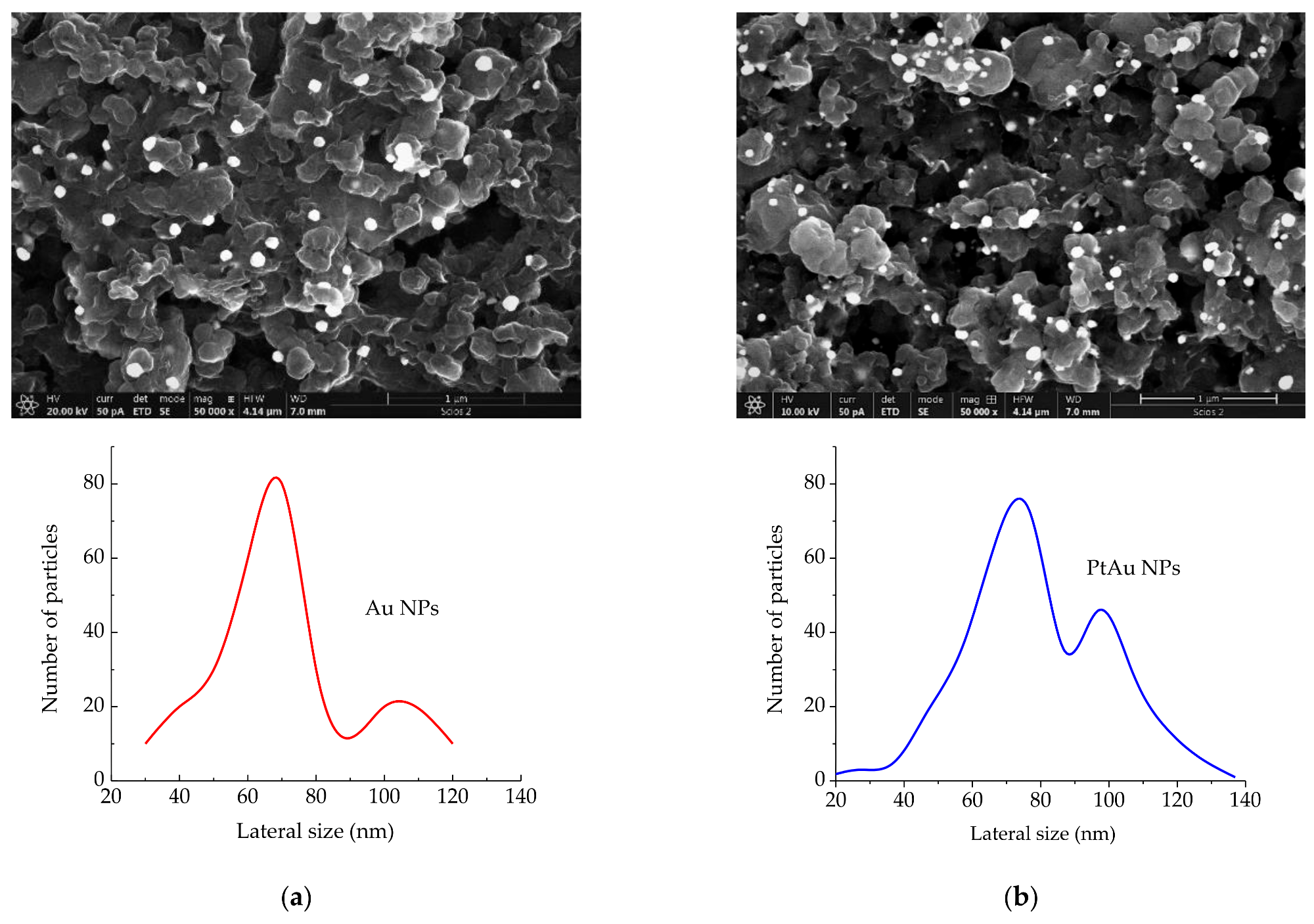
SEM images (magnification = 50,000×, scale-bar = 1 μm) of: (a) Au/rGO and Au NPs size distribution; (b) PtAu/rGO and PtAu NPs size distribution. © Rakočević, L., Simatović, I. S., Maksić, A., Rajić, V., Štrbac, S., & Srejić, I. (2022)
Graphene to the Rescue
Graphene is among the carbon-based substances that has piqued the curiosity of many scientists in recent years. Because of its intriguing two-dimensional structure, graphene has remarkable electromagnetic, mechanical, and optical characteristics. Consequently, graphene and its derivatives, reduced graphene oxide (rGO) and graphene oxide (GO), have proven to be useful as catalyst supports for HER and a variety of other processes.
Keeping this in mind, the authors chose to investigate two materials for the purpose of effectively catalyzing the HER; Au/rGO and PtAu/rGO. Both of which are deposition of metals on graphene derivatives support.
It is worth noting that the atomic percentage of both gold and platinum is very less (0.3% and 0.6% respectively) as compared to pure PtAu compounds, thus making the whole catalyst available at a fraction of original cost while retaining most of the desirable properties of the catalyst for hydrogen evolution reactions.
The Stringency of Scientific Method
The electrochemical impedance spectroscopy (EIS) and linear sweep voltammetry (LSV) were used to evaluate the HER activity of both catalysts produced.
Chronoamperometry (CA) had been utilized to assess the durability of PtAu/rGO, which had been highly active throughout the reaction. Using independent measurements, the evolution of hydrogen on PtAu/rGO and Au/rGO was also examined. The experiments included unprompted deposition of Gold and Platinum at the boundaries of graphene.
The PtAu/rGO electrode demonstrated exceptional HER activity, with the baseline potential being relatively close to that of equilibrium potential for HER. This means that PtAu/rGO is very well suited to be an economical replacement of a simple Pt catalyst for HER in sulfuric acid solutions.
Important Takeaways
Using PtAu/rGO as a catalyst for the evolution of hydrogen gas in a solution of sulfuric acid, the authors showed the outstanding catalytic activity of the catalyst.
Scanning Electron Microscope (SEM) images revealed that PtAu nanoparticles were randomly scattered and uneven in size, and that they were concentrated largely on the borders of rGO sheets. Both Energy Dispersive X-Ray Spectroscopy (EDS) and X-Ray Photoelectron Spectroscopy (XPS) examination indicated the low atomic percentages of gold and platinum in the samples. Furthermore, the LSV profile for the PtAu/rGO electrode demonstrated an exceptional onset latent for HER and a low-slung Tafel slope, both of which are exceptional for this kind of electrode. The findings of the EIS studies are consistent with the LSV data, demonstrating a high level of HER activity.
The CA measurement, which was carried out for 40 minutes at a constant electrode voltage, demonstrates that the PtAu/rGO catalyst for hydrogen evolution has excellent stability and endurance.
This discovery potentially opens the door to economical fuel generation using hydrogen evolution.
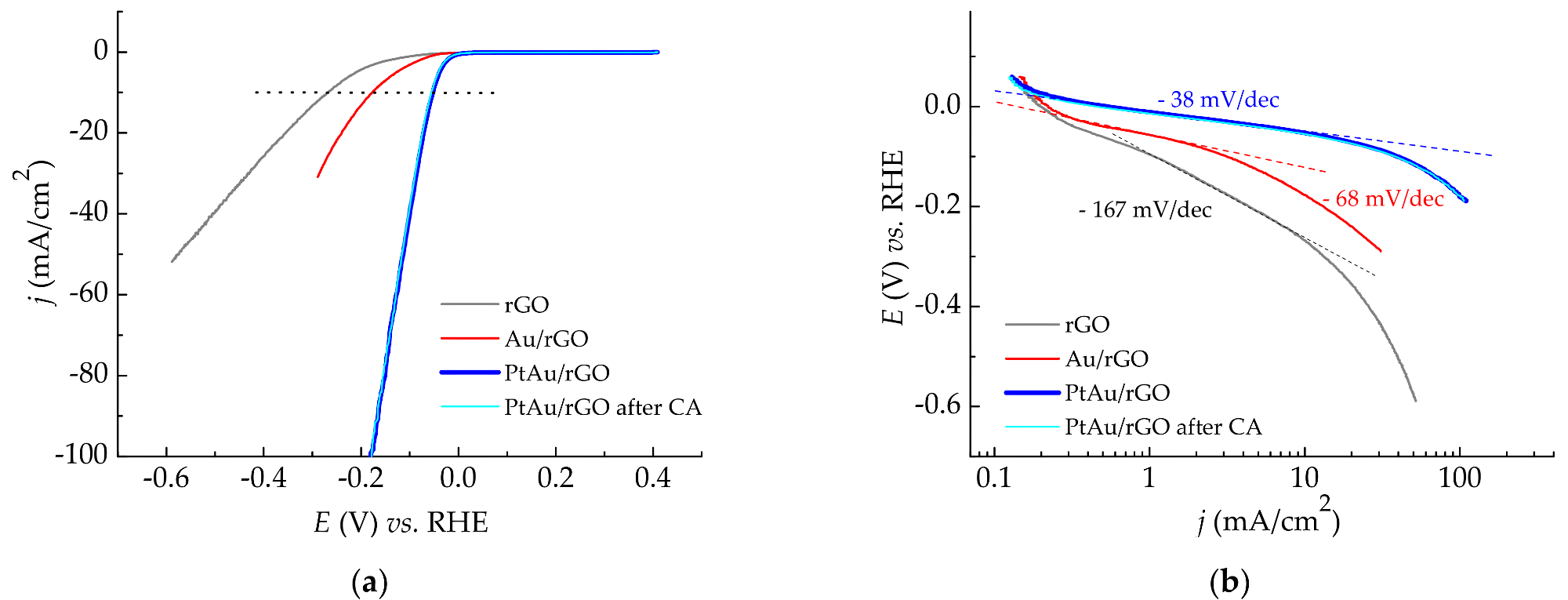
Hydrogen evolution reaction in 0.5 M H2SO4: (a) LSV curves recorded at a scan rate of 10 mV/s for rGO, Au/rGO, and PtAu/rGO electrodes, as well as for PtAu/rGO alone after stability test; (b) Derived Tafel slopes. © Rakočević, L., Simatović, I. S., Maksić, A., Rajić, V., Štrbac, S., & Srejić, I. (2022)
Continue reading: How are Nanocatalysts Used for Environmental Applications?
Reference
Rakočević, L., Simatović, I. S., Maksić, A., Rajić, V., Štrbac, S., & Srejić, I. (2022). PtAu Nanoparticles Supported by Reduced Graphene Oxide as a Highly Active Catalyst for Hydrogen Evolution. Catalysts, 12(1). Available at: https://www.mdpi.com/2073-4344/12/1/43
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.