A group of researchers synthesized, evaluated, and used environmental, highly permeable, and economical carbon dots as a corrosion inhibitor in a 15 % HCL solution, according to a study published in the Journal of Physics and Chemistry of Solids.
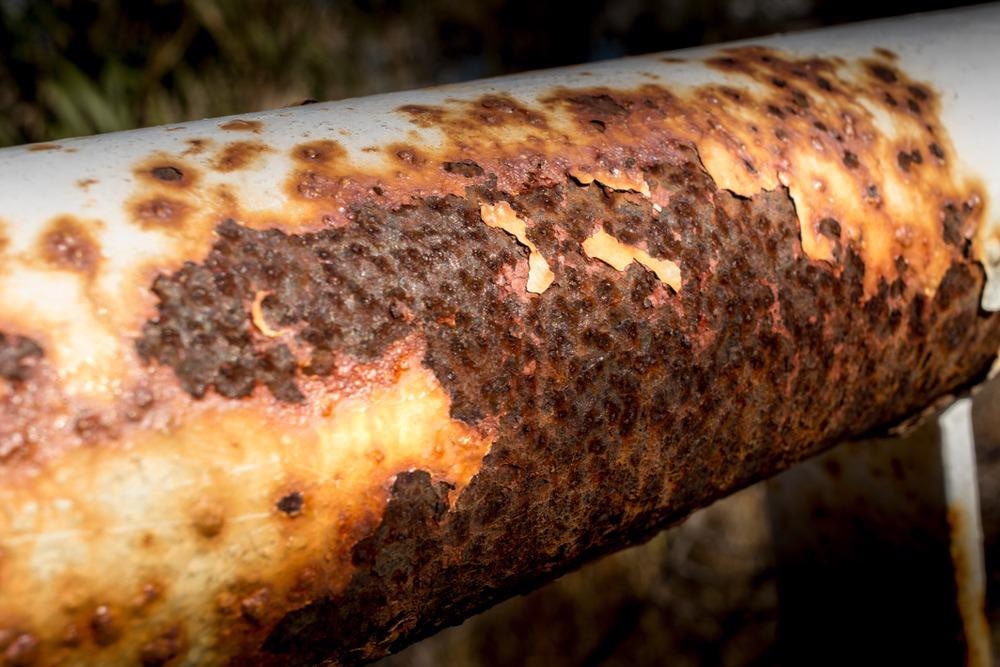
Study: Novel carbon dots as efficient green corrosion inhibitor for mild steel in HCl solution: Electrochemical, gravimetric and XPS studies. Image Credit: Guilbaud Stan/Shutterstock.com
Infrared radiography, ultraviolet-visible spectroscopy, and fluorescence spectroscopy were used to evaluate both inhibitors.
Weight loss, electrolytic impedance spectrometry, and electrochemical impedance spectroscopy measurements were used to assess the inhibitory impact of carbon dots.
Electron microscopy, atomic force microscopy, and XPS analyses were used to examine the surface topography of unconstrained and hindered MS samples by the researchers.
Corrosion Complications of Mild Steel
Mild steel is widely utilized in a variety of manufacturing industries owing to its varied physical features and low cost, but MS corrosion is among the most important issues that companies face.
Corrosion causes substantial costs for re-establishment, replacement of different constituents and apparatus, casualties, and environmental issues. Because it eliminates accumulated salts and layers in pipes, most industrial procedures, such as fuel drill acidization to boost crude petroleum oil output, are performed by flowing 15 percent HCl solution across steel pipes.
The aggressiveness of a 15 percent HCl solution, on the other hand, produces significant corrosion and deterioration to steel, degrades its capabilities, and restricts its usage.
As a result of all of these concerns, researchers focused on the corrosion challenge and devised strategies to extend the life of infrastructures, equipment, and metallic gadgets.
Utilization of Corrosion Inhibitors
Different anticorrosion strategies, such as enhancing the materials, adding inhibitors, utilizing different kinds of coatings, and changing the atmosphere, may considerably reduce the deterioration of metallic elements.
The use of corrosion inhibitors is the simplest and cheaper strategy for corrosion prevention among these possibilities.
Corrosion inhibitors prevent degradation by adhering to the surface of the metal and have excellent anti-corrosive properties.
Traditional Corrosion Inhibitors and their limitations
In an acidic medium, substances having molecules such as O, N, S, and radicals in their molecule be excellent corrosion inhibitors. These compounds are readily deposited on the metallic surface by the quasi electrons contained in the moieties, reducing the metal's interfacial interaction with the corrosion environment.
As a consequence, deterioration is suppressed by obstructing the surface's active areas.
Corrosion inhibitors are frequently used to treat corrosion in acidic conditions in a variety of industries. The petroleum extraction sector, oil refining business, chemical plants, huge manufacturing industrial sector, water filtration industry, and additive product sector are the key industries that use corrosion inhibitors.
Numerous chemical compounds have been described as corrosion inhibitors in the past, however, the majority of them are poisonous and only effective at greater concentrations.
Scientists are looking for anti-corrosive chemicals that are non-toxic, sustainable, stable, and have high inhibitory effectiveness due to growing awareness of safety and environmental contamination.
Carbon Dots as Corrosion Inhibitors
Carbon dots (CDs) have attracted a lot of interest because of their unusual features, such as nontoxicity, high solvent dispersion, and bioactivity.
Carbon dots are used in the detection, optoelectronics, bioimaging, healthcare, catalysts, and corrosion inhibition, among other things.
The researchers in this study synthesized and characterized several novel carbon dots with sufficient photoactivity to investigate their anti-corrosive activity for mild steel, taking into consideration the decent catalytic properties, sustainability, and permeability of carbon dots documented by other researchers.
Malononitrile, ammonia, and 5-hydroxy isophthalic anhydride were utilized as starting materials to fabricate two new carbon dots, CDMH and CDMU.
Research Findings and Conclusion
CDMU and CDMH were found to be good water-soluble corrosion inhibitors for MS in a 15% HCl solution.
The carbon dots were evaluated using FTIR spectroscopy, UV-VIS spectroscopy, fluorescence spectroscopy, X-Ray photonic spectrometry, and electron microscopy. The usual diameters of CDMU and CDMH were 2.04 and 2.52 nm, accordingly.
The inhibitory efficacy of CDMU and CDMH at 100 mg/L was found to be 97 and 90%, respectively, at roughly 300 K.
CDMU is a better inhibitor than CDMH because of the higher number of active centers. A higher concentration of carbon dots and higher temperature resulted in better protective capability due to strong adhesion, and weaker shielding ability due to ablation, the Langmuir isotherm model was followed by CDMU and CDMH deposition on MS surface.
According to EIS, an increase in the concentration of CDMU and CDMH resulted in increased electrical resistivity, while drop-in pseudocapacitance, indicated adsorption of the studied inhibitor on the MS surface.
Reference
V, Saraswat., Kumari R., and Yadav, M.(2022). Novel carbon dots as efficient green corrosion inhibitor for mild steel in HCl solution: Electrochemical, gravimetric and XPS studies. Journal of Physics and Chemistry of Solids. Available at: https://www.sciencedirect.com/science/article/abs/pii/S0022369721004078
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.