A work that presents a direct inspection and measurement of the basic process of lithium infiltration in Li6PS5Cl (LPS) solid-state electrolyte has been posted to Research Square* preprint server.

Study: Nano-scale mechanism of crack nucleation/propagation and lithium penetration in solid electrolyte. Image Credit: cybrain/Shutterstock.com
The Need for Next-Generation Energy Storage Technology
Despite the fact that lithium-ion batteries (LIBs) dominate the present energy-storing industry, their capability is approaching theoretical limitations. As a result, there is a pressing necessity for the production of new energy-storing technologies that can fulfill the demands of high-demand applications like electric cars.
Owing to its great hypothetical capability and small electrochemical potential in comparison to the normal hydrogen-based electrode, the use of a Li anode is vital. Nonetheless, increasing energy density creates safety issues about unforeseen incidents, and using a Li anode enhances the danger of dendrite nucleation and development, which might lead to short-circuiting, fire hazards, or even explosions.
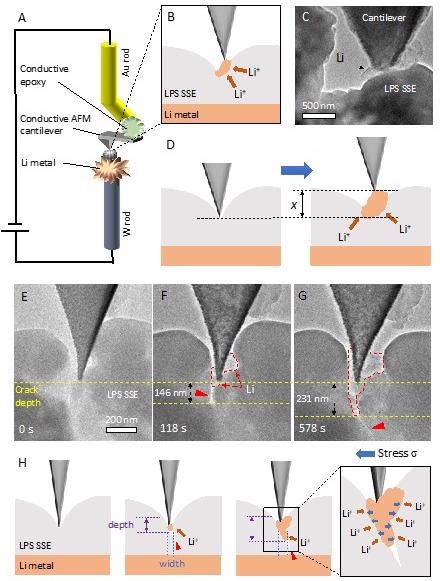
(A) Schematic illustration of the in situ TEM setup showing the AFM cantilever attached to a gold rod via a conductive epoxy approaching the SSE on lithium metal scratched onto a tungsten rod. (B) Magnified view of cantilever approaching the SSE. (C) TEM image of cantilever tip contacting LPS SSE showing lithium growth. (D) Schematic of cantilever displacement due to lithium dendrite growth upon biasing. (E-G) TEM snapshots from video shown at different timestamps. Initially the cantilever and LPS SSE were aligned to obtain contact. At 118s Li plating has occurred and the crack has started to open. At 578s Li plating has continued to widen the crack. Scale bar is 200 nm. (H) Schematic showing the crack increasing in both the depth and width directions, and the stress from the lithium pushing the crack apart as Li is deposited at the Li/SSE interface. © Diaz, M., Tu, Q., and Kushima, A. (2022, February 18).
With their prospects of improved energy density and better safety, all-solid-state lithium batteries (ASSLBs) provide an answer in this case.
Employing a solid-state electrolyte (SSE) with a substantially greater modulus than Li is intended to minimize dendrite entry and enable safe usage of a high potential Li anode.
Pros and Cons of Using LPS
A potential solid electrode material for ASSLBs is Li2S-P2S5 based sulfide (LPS).
At ambient temperature, it has a strong Li+ conductance, poor electron conduction, and a broad electrochemical stability range against Li. As far as Li dendrites are concerned, LPS is no exception.
Although squeezing the SSE against the electrode is required for optimal connection in ASSLBs, that compressive stress may also be causing crack formation when lithium is driven into the pores of LPS.
Crack propagation is most likely in fault regions of the LPS SSE, as well as in locations where LI plating and stripping happen regularly.
As LPS has been demonstrated to have poor fracture toughness and to be highly brittle, lithium plating in surface flaws is a significant reason for worry. Furthermore, fractures in SSEs have been demonstrated to reduce ionic conduction, reducing one of LPS's key advantages.
It is therefore critical to have a better understanding of the function of lithium in the nucleation and propagation of fractures in LPS SSEs.
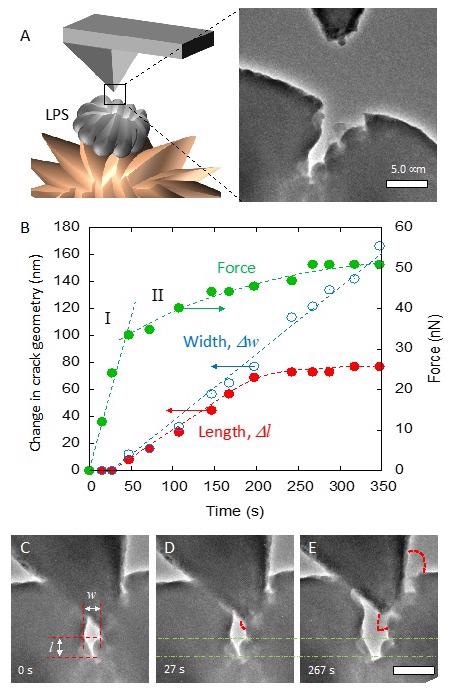
(A) Schematic illustration of conducting cantilever approaching a defect on LPS surface (left) and the TEM image (right). (B) Force evolution and change in the crack geometry during the initial lithium plating and crack growth. The force necessary for crack growth was 34nN. (C) Initial alignment of the cantilever inside the crack. Dimensions of the crack width “w” and length “l” are labeled. (D) After 27s, the crack began to open. (E) Crack growth and lithium deposition is easily seen after 267s. Outline of the plated lithium is indicated by the dashed lines. The dash-dotted lines indicate the initial crack length, l, from B as a reference, and the scale bar is 500 nm. © Diaz, M., Tu, Q., and Kushima, A. (2022, February 18).
Reason Behind Crack Propagation
Cracks created by Li plating induce further Li plating in that location, which boosts local electron transport, lowers the energy threshold, and promotes dendrite formation and spreading.
The threshold force required to commence crack development, as noted in the paper, is substantially smaller than the peak force of dendrites developing through the LPS SSE, indicating exactly how effortless it is for dendrites to originate in a defective region of the SSE and propagate to establish a rapid dendrite development region.
Important Takeaways
An on-site TEM analysis was carried out in this study to directly view and investigate the nucleation/propagation processes of nanosized fractures in an SSE, giving clear confirmation of Li penetration in SSEs owing to nanoscale flaws.
During Li plating, no visible deformation of the LPS was seen in sections of the LPS SSE where no large surface fractures were found.
Upon electroplating Li in an already present imperfection on the LPS SSE surface, it was able to exert enough stress on the surrounding LPS to expand the crack.
Lithium grown in fault locations required less force to induce fracture spreading than lithium deposited at flawless regions. It should be noted that no external tension was necessary for the Li to force open the crack and spread into the LPS.
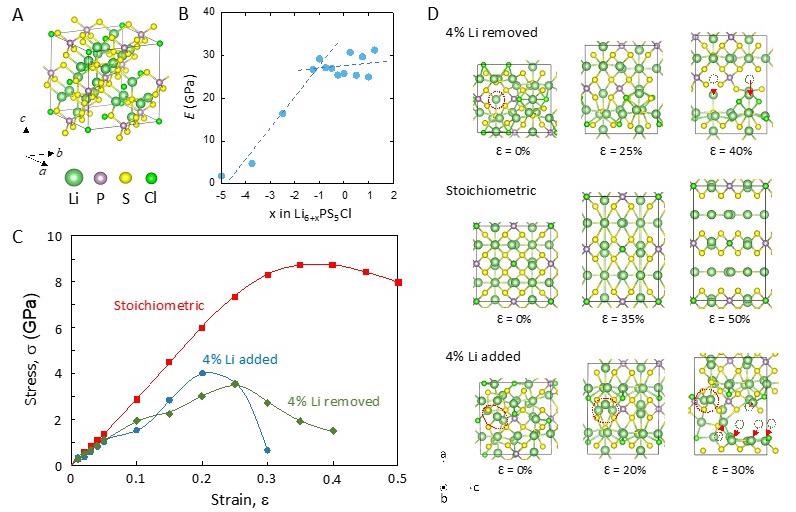
(A) Simulation mode of Li6PS5Cl. (B) Modulus vs. Li concentration of LPS SSE. (C) The stress-strain curve of the LPS with stoichiometric and ±4% Li concentrations. The yield stress drops significantly upon a 4% deviation from the stoichiometric lithium amount. (D) Structural changes of LPS during the tensile deformation at different Li concentrations. © Diaz, M., Tu, Q., and Kushima, A. (2022, February 18).
The quantitative examination of the applied stress to the fracture wall during Li plating indicated a barrier force at which the crack would open.
The findings of this research highlight the significance of reducing the number of imperfections on the SSE surface, which may serve as beginning locations for crack propagation and lithium penetration.
*Important Notice
Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information
Reference
Diaz, M., Tu, Q., and Kushima, A. (2022, February 18). Nano-scale mechanism of crack nucleation/propagation and lithium penetration in solid electrolyte. PREPRINT Available at: https://www.researchsquare.com/article/rs-1346996/v1
Further Reading
Aurbach, D., Zinigrad, E., Cohen, Y., & Teller, H. (2002). A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ionics, 405–416. https://www.sciencedirect.com/science/article/pii/S0167273802000802
Whittingham, M. S. (2012). History, Evolution, and Future Status of Energy Storage. Proceedings of the IEEE, (pp. 1518–1534). https://ieeexplore.ieee.org/document/6184265
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.