Prof. Hong Zhang and Dr. Yansong Feng at the Van 't Hoff Institute for Molecular Sciences at the University of Amsterdam (UvA) have engineered and synthesized unique multi-layered, multi-functional nanoparticles that allow radiotherapy and photodynamic therapy to be combined for deep cancer tissue.
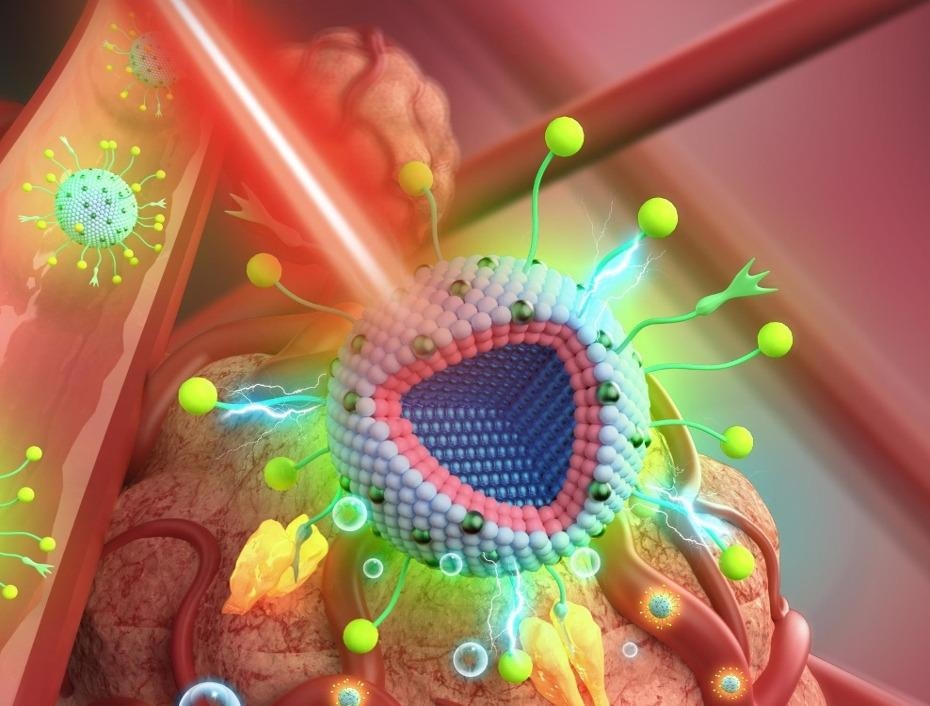 Artist's impression of applying a 20-nanometer multi-layered nanoparticle for therapy of deep cancer tissue. When injected into the body, the particles attach to the tumor site and assist in localization and therapy. Image Credit: University of Amsterdam.
Artist's impression of applying a 20-nanometer multi-layered nanoparticle for therapy of deep cancer tissue. When injected into the body, the particles attach to the tumor site and assist in localization and therapy. Image Credit: University of Amsterdam.
A preliminary pre-clinical assessment of the particles has shown their therapeutic prospect. There is currently a pending patent, and the university is currently looking for partners for additional development or licensing.
What sets the nanoparticles apart is that they allow radiotherapy and photodynamic therapy to be integrated while using just X-Rays. The particles also enable imaging of deep tissue, facilitating the image-guided targeting of the combined therapy.
Combined Therapy
In photodynamic therapy, visible light is employed to trigger photosensitizers that discharge radical oxygen species to kill cancer cells. It attacks various areas of a cancer cell compared to traditional radiotherapy that employs X-Rays. The collective use of both therapies enhances the destruction of tumorous tissue and regularly decreases the mandatory X-Ray dose.
However, since photodynamic therapy is driven by light, it is hard to use it to treat cancer tissue positioned deep within the body. To do so necessitates an invasive procedure, for example, endoscopy using an optical fiber. With X-Rays, there is no such issue. They effortlessly penetrate the body and are directed in such a manner that they can achieve their devastating work at the tumor region.
By engineering nanoparticles that can discharge visible light upon irradiation using X-Rays, scientists at UvA have currently discovered a method to apply photodynamic therapy at deep areas without the need for invasive procedures. The particles were engineered during the Ph.D. research of Dr. Yansong Feng, directed by Prof. Hong Zhang at the Molecular Photonics research group (UvA).
Image-Guided Targeting
The nanoparticle contains a core enclosed by two outer layers. The outermost layer performs scintillation — a method that turns X-Rays into visible light and thereby enabling photodynamic therapy at any area accessible by radiation therapy. The second layer acts as a buffer layer that energetically separates the scintillating layer from the nanoparticle core.
Within the core, the scientists applied another crucial therapy-improving feature. It is adept at upconversion luminescence which means it can alter the frequency of light. The team tweaked the upconversion in such a manner that the nanoparticle discharges a red visible light upon irradiation with X-Rays or near-infrared (NIR) radiation. In this manner, the researchers could effectively facilitate image-guided therapy.
On irradiation with NIR, which has a fairly long penetration depth, the particles obtain a bright red color, thus exposing the tumor’s location. The core continues to discharge red light during radiotherapy using X-Rays, though at a lower concentration. The produced red light does not inhibit the photodynamic therapy.
Positive Preclinical Evaluation
As a proof of concept, the team examined the behavior of the nanoparticles in cancer treatment research with mice (in vivo) and cell cultures (in vitro). This offered a vivid indication of the therapeutic and safety prospect of the particles.
Together with Innovation Exchange Amsterdam (IXA, the university's technology transfer office), the scientists are currently seeking licensees and/or partners to further advance this new method into a commercially feasible application, which would comprise the completion of preclinical trials and boost entry into full clinical trials.
This would be key in proving the safety of the nanoparticles, their performance during therapy, their ease of use and the total effectiveness of their application.
Journal Reference:
Feng, Y., et al. (2022) Scintillating Nanoplatform with Upconversion Function for Synergy of Radiation and Photodynamic Therapies of Deep Tumor. Journal of Materials Chemistry C 10. doi.org/10.1039\D1TC04930E.