As an alkali metal electrode for emerging electrolytic energy storing devices, potassium (K) is an attractive substitute for lithium. Unfortunately, potassium utilization is hampered by dendrite formation and volumetric fluctuations during cycling.

Study: Codoped porous carbon nanofibres as a potassium metal host for nonaqueous K-ion batteries. Image Credit: Troggt/Shutterstock.com
A study published in the journal Nature Communications proposed the fabrication and employment of highly porous nitrogen (N) and zinc (Zn) codoped carbon nanofibers as potassium hosts to avoid these problems.
K-Ion Batteries
Lithium-ion batteries (LIBs) have the disadvantages of lithium scarcity and high extracting costs, which limit their utilization for storage.
K-ion batteries are the subject of increasing interest as a low-cost alternative to LIBs. Potassium is a virtually inexhaustible natural resource and is thermodynamically stable. As a result, potassium has great prospects for scalable electrochemical energy storage applications.
It is projected that K-ion batteries can have greater operational voltages than those of their competitors, including LIBs.
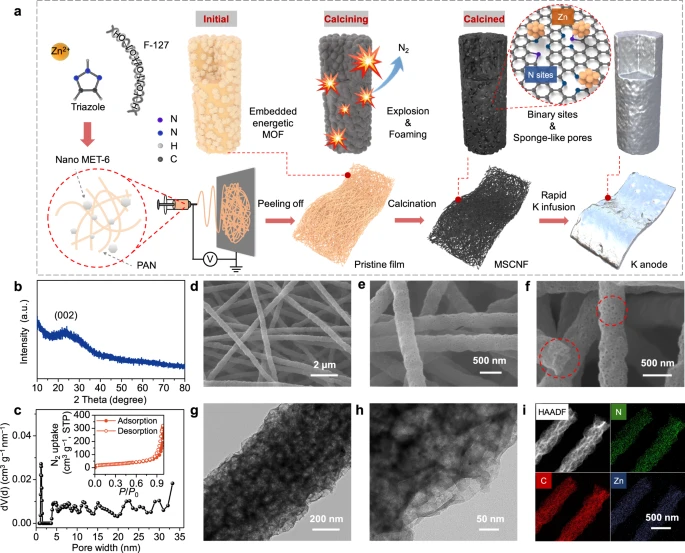
Figure 1. Synthesis and physicochemical characterization of MSCNFs. a Synthetic route of MSCNFs and the corresponding composite K anode. b PXRD patterns of MSCNFs. c Pore distribution of MSCNFs and the corresponding N2−77 K adsorption isotherms (inset). d–f SEM images of MSCNFs. The red dashed lines highlight the porous structure of the MSCNFs. g TEM image and h HRTEM image of MSCNFs. i EDS elemental mapping of MSCNFs in HAADF mode.© Li, S., Zhu, H. et al. (2022).
Advantages of Potassium Anodes in K-Ion Batteries
Various materials have been explored for use as an anode in K-ion batteries. Potassium metal is the ideal option because of its minimum redox potential and high specific capacity compared with other anode material choices.
Furthermore, the inclusion of potassium metal may enable the use of potassium-free cathodes in fabricating K-ion batteries with high specific energies.
The Problem of Dendrite Growth
Unfortunately, anodes of potassium metal, like those of lithium and sodium, suffer from dendrite formation during electrolytic plating and stripping. This phenomenon greatly limits their practical application in K-ion batteries.
Exposure to potassium dendrites with large surfaces may lead to continuous reactions with the electrolyte, resulting in poor Coulombic efficiency. Furthermore, the stripping mechanism typically takes place at the dendritic roots, which causes electrical isolation of the dendrites, producing "dead potassium".
Dendrites may also form on the opposing electrode, causing interior short circuits, failure of K-ion batteries, and even safety hazards like explosion or fire.
Generally, the volatile solid electrolyte interphase (SEI) is the primary source of potassium dendrites.
Methods for Suppressing Potassium Dendrites
By modifying the surface of the metal or solid electrolyte interphase, electrolytic compositions or electrode surface design may effectively prevent the formation of potassium dendrites.
While a better SEI may guard the potassium anode against dendrite growth to some degree, this protective layer is rendered useless when the electrode suffers a significant volume fluctuation during potassium plating and stripping.
A conductive host matrix can also be used to stabilize the potassium anode.
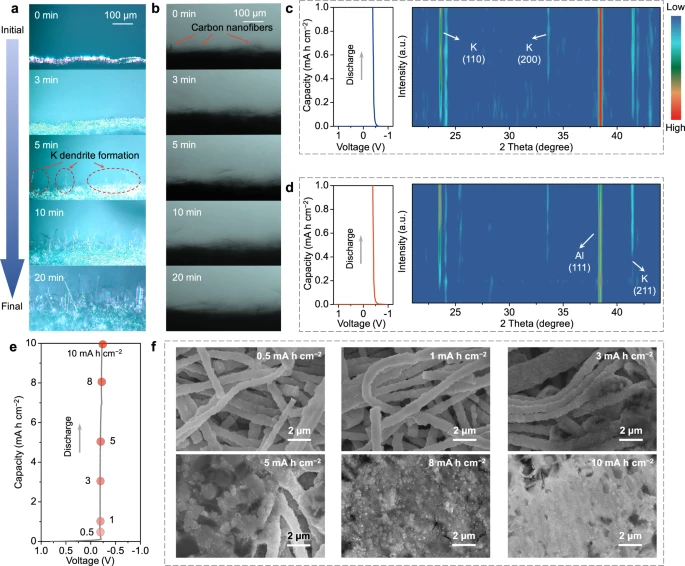
Figure 2. Physicochemical investigation of potassium metal deposition for various potassium metal hosts. In situ optical microscopy observation of K deposition on a Cu foil and b MSCNFs at a current density of 6 mA cm−2. Contour plots of operando XRD patterns for K deposition on c Al foil and d MSCNFs at a current density of 0.5 mA cm−2. e Voltage profiles of K deposition on MSCNFs at a current density of 0.5 mA cm−2 and f the corresponding SEM images of deposited MSCNFs at different discharge states. All electrochemical measurements were carried out at a temperature of 25 ± 2 °C. © Li, S., Zhu, H. et al. (2022).
Benefits of Using Conductive Hosts to Stabilize Potassium Anodes
Using a conductive host allows for the encapsulation of potassium metal inside the host matrix, reducing the possibility of unwanted reactions between the electrolyte and the alkali metal.
Furthermore, by mitigating volumetric changes, a host matrix may increase the structural integrity of the potassium anode, offer conducting networks that promote the quick transference of ions and electrons and lower the localized current density, thereby impeding dendrite formation.
Metallic hosts are difficult to use due to their large density and limited space usage. Carbonaceous host materials, on the other hand, are better suited to accommodate potassium thanks to their low weight and electrolytic stability, which may promote greater specific energy and energy densities.
Carbon nanofibers that are both robust and electrically conductive show great promise for potassium accommodation in K-ion batteries. Therefore, combining carbon nanofibers with various potassiophilic substances is a viable approach for improved potassium plating/stripping electrochemistry.
Metallic nanoparticles are also of interest because of their strong potassium affinity and ease of production. Gold, silver, and zinc nanoparticles have already been shown to be beneficial in promoting heterogeneous seeded development of lithium on carbon nanofiber hosts.
The use of metallic nanoparticles capable of creating potassium alloys represents an effective technique for increasing the potassiophilicity of carbon nanofiber hosts.
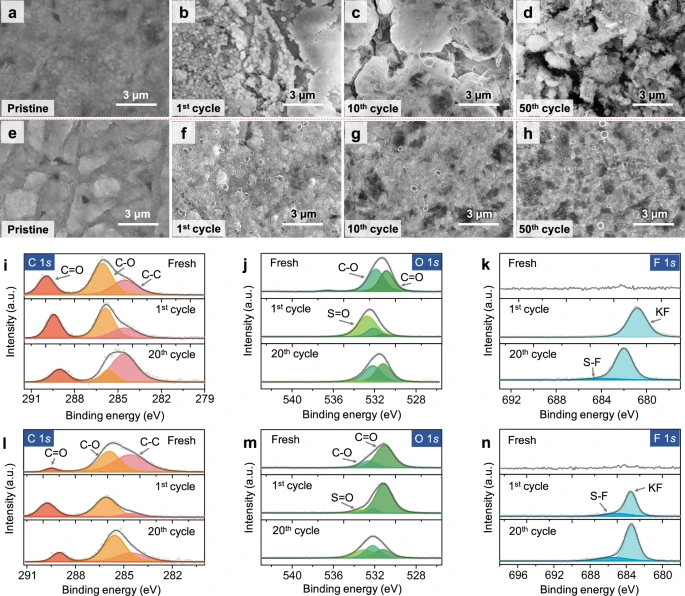
Figure 3. Ex situ physicochemical characterizations of various potassium metal-based electrodes. Ex situ SEM images of a–d bare K and e–h MSCNF-K anodes from symmetric cells at different cycle numbers. Ex situ XPS spectra of i C 1 s, j O 1 s and k F 1 s from symmetric cells based on Cu-K anodes at different cycle numbers (Fresh, after the 1 st charge, and after the 20th charge). Ex situ XPS spectra of l C 1 s, m O 1 s and n F 1s from symmetric cells based on MSCNF-K anodes at different cycle numbers (Fresh, after the 1st charge, and after the 20th charge). The ex situ electrodes were sampled at an intermediate state of potassiation. All electrochemical measurements were carried out at a temperature of 25 ± 2 °C. © Li, S., Zhu, H. et al. (2022).
Main Findings of the Study
In this work, the team produced and subsequently characterized hierarchically porous carbon nanofibers containing monodispersed binary active spots and nitrogen and zinc clusters.
The porous carbon nanofiber host, combined with zinc-containing binary potassiophilic areas, offered strong potassiophilic nature, resulting in greater potassium atom nucleation, low weight, and ample room for potassium metal accommodation.
Using the carbon nanofiber host, efficient induction of a homogenized electrical field and smooth potassium plating were also achieved.
The developed carbon nanofiber and potassium composite anodes, having a large potassium loading content and specific discharge capability, may be manufactured using a quick thermal infusion technique.
In symmetric cell and K-PPB and K-S full cell testing, the developed carbon nanofiber and potassium composite electrodes retained excellent rate capability and cycle stability.
Reference
Li, S., Zhu, H. et al. (2022). Codoped porous carbon nanofibres as a potassium metal host for nonaqueous K-ion batteries. Nature Communications, 13. Available at: https://www.nature.com/articles/s41467-022-32660-y
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.