Reviewed by Emily Henderson, B.Sc.Nov 3 2022
Researchers from the Université de Montréal in Canada have created and verified a new class of DNA-based drug transporters that are 20,000 times smaller than a human hair and could enhance cancer treatment and other diseases.
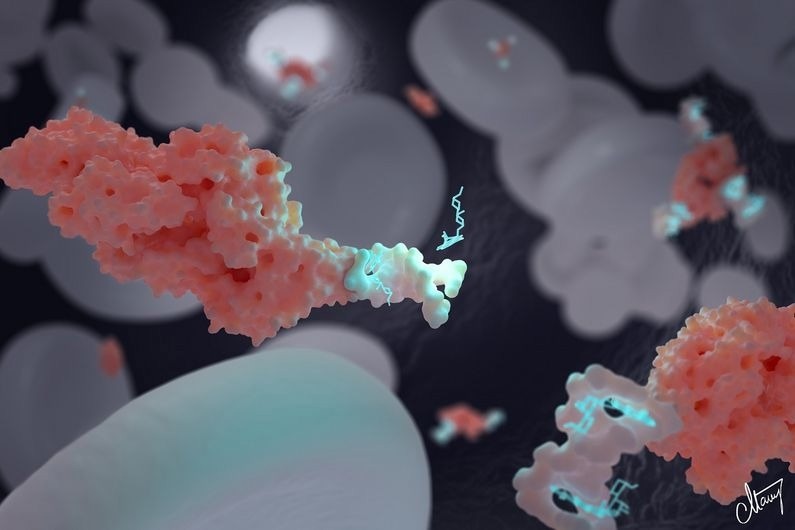 The DNA-based nanotransporter developed by Alexis Vallée-Bélisle and his team can transport and deliver precise concentrations of drugs: in this picture, doxorubicin, a chemotherapeutic drug. These nanotransporters can also be attached to specific biomolecules to optimize drug distribution. Here, we see a nanotransporter (white) attached to albumin (pink) to maintain doxorubin (light blue) in blood circulation. Image Credit: MONNEY MEDICAL MEDIA / CAITLIN MONNEY
The DNA-based nanotransporter developed by Alexis Vallée-Bélisle and his team can transport and deliver precise concentrations of drugs: in this picture, doxorubicin, a chemotherapeutic drug. These nanotransporters can also be attached to specific biomolecules to optimize drug distribution. Here, we see a nanotransporter (white) attached to albumin (pink) to maintain doxorubin (light blue) in blood circulation. Image Credit: MONNEY MEDICAL MEDIA / CAITLIN MONNEY
According to a recent study published in Nature Communications, these molecular transporters can be chemically engineered to deliver drugs in the best concentration possible, outperforming existing techniques.
Optimal Dosing at All Times: A Medical Challenge
Providing and maintaining a therapeutic drug dosage during treatment is one of the most important factors in the successful treatment of disease. Overexposure raises side effects whereas sub-optimal therapeutic exposure decreases effectiveness and often results in drug resistance.
Modern medicine continues to struggle with maintaining the ideal drug concentration level in the blood. Patients must take numerous doses at regular intervals since most drugs degrade quickly, and they frequently forget to do so. The drug concentration in each patient’s blood also varies considerably because each has a unique pharmacokinetic profile.
Alexis Vallée-Bélisle, an Associate Professor of Chemistry at the Université de Montréal and a specialist in bio-inspired nanotechnologies, began to investigate how biological systems control and maintain the concentration of biomolecules after noticing that only about 50% of cancer patients receive the ideal drug dosage during certain chemotherapy.
We have found that living organisms employ protein transporters that are programmed to maintain precise concentration of key molecules such as thyroid hormones, and that the strength of the interaction between these transporters and their molecules dictates the precise concentration of the free molecule.
Alexis Vallée-Bélisle, Associate Professor, Chemistry, Université de Montréal
This simple and direct notion prompted Valléé-Belisle and his research group to create synthetic drug transporters that mimic the natural effect of preserving a precise drug concentration during treatment. Valléé-Belisle holds a Canada Research Chair in bioengineering and bionanotechnology.
Arnaud Desrosiers, a Ph.D. student at UdeM, is the study’s first author. He discovered and created two DNA transporters: one for the antimalarial quinine and the other for the chemical doxorubicin, frequently used to treat leukemia and breast cancer.
He went on to show how these synthetic transporters could be easily set up to deliver and maintain any desired drug concentration.
More interestingly, we also found that these nanotransporters could also be employed as a drug reservoir to prolong the effect of the drug and minimize its dosage during treatment. Another impressive feature of these nanotransporters is that they can be directed to specific parts of the body where the drug is most needed - and that, in principle, should reduce most side effects.
Arnaud Desrosiers, Study First Author and PhD Student, Université de Montréal
Nanotreated Mice: Reduced Cardiotoxicity
The researchers collaborated with Jeanne Leblond-Chain, a pharmacist at the Université de Bordeaux in France, Luc DesGroseillers, a biochemist at the Université de Montréal, Jérémie Berdugo, a pathologist at the Université de Montréal, Céline Fiset, a pharmacist at the Montreal Heart Institute, and Vincent De Guire, a clinical biochemist at the Maisonneuve-Rosemont Hospital, which is affiliated with Université de Montréal, to show the effectiveness of these nanotransporters.
The team found that a particular drug-transporter formulation enables doxorubicin to be kept in circulation and significantly inhibits its diffusion toward important organs like the heart, lungs, and pancreas.
This formulation kept doxorubicin in the blood of mice 18 times longer than usual and minimized cardiotoxicity, keeping the mice healthier as indicated by their normal weight gain.
Vallée-Bélisle stated, “Another great property of our nanotransporters is their high versatility. For now, we have demonstrated the working principle of these nanotransporters for two different drugs. But thanks to the high programmability of DNA and protein chemistries, one can now design these transporters to precisely deliver a wide range of therapeutic molecules.”
He further added, “Additionally, these transporters could also be combined with human-designed liposomic transporters that are now being employed to deliver drugs at various rates.”
A Clinical Study for Blood Cancers?
The scientists are now eager to confirm if their discovery works clinically. They believe their doxorubicin nanotransporter could be useful in treating blood cancers since it is designed to keep the drug in blood circulation as effectively as possible.
“We envision that similar nanotransporters may also be developed to deliver drugs to other specific locations in the body and maximize the presence of the drug at tumor sites. This would drastically improve the efficiency of drugs as well as decrease their side effects.”
The National Science and Engineering Research Council of Canada, the Canada Research Chairs, Les Fonds de recherche du Québec - Nature et technologies, and Le regroupement québécois de research sur la fonction, l’ingénierie et les applications des protéines (PROTEO) provided funding for this study.
Journal Reference:
Desrosiers, A., et al. (2022) Programmable self-regulated molecular buffers for precise sustained drug delivery. Nature Communications. doi:10.1038/s41467-022-33491-7.