.jpg) By Pritam RoyReviewed by Susha Cheriyedath, M.Sc.Nov 21 2022
By Pritam RoyReviewed by Susha Cheriyedath, M.Sc.Nov 21 2022In an article published in PNAS, researchers introduced the magnetotactic bacteria (MTB) Mms6 proteins into a reverse micelle structure to create a nanoreactor that resembled a magnetosome. This magnetosome-inspired nanoscale chamber synthesized a single domain’s magnetic nanoparticles (MNPs).
Study: Magnetosome-inspired synthesis of soft ferrimagnetic nanoparticles for magnetic tumor targeting. Image Credit: peterschreiber.media/Shutterstock.com
Subsequently, their magnetic characteristics and morphology were analyzed and contrasted with those of the naturally occurring magnetosomes created by AMB-1 MTB.
One of the most effective methods for enhancing the targeting efficacy of magnetic nanodrug carriers utilizing an external magnetic field to reach their targets is magnetic targeting. The magnetosome, a unique “organelle” created by biomineralization in MTB, is a naturally occurring MNP of biological origin. It is crucial for MTB magnetic navigation to react to geomagnetic fields.
The magnetism and crystal morphology of the MNPs formed through thermal decomposition were identical to those of natural magnetosomes, but the particles were smaller. Thus, the synthesized magnetosome-like MNPs penetrated a tumor mouse model's lesion area with good monodispersion. This monodispersion increased tumor penetration by order of magnitude due to their powerful magnetic targeting ability created by soft ferromagnetism. Hence, the improved tumor penetration resulted in a positive contrast in the tumor spots.
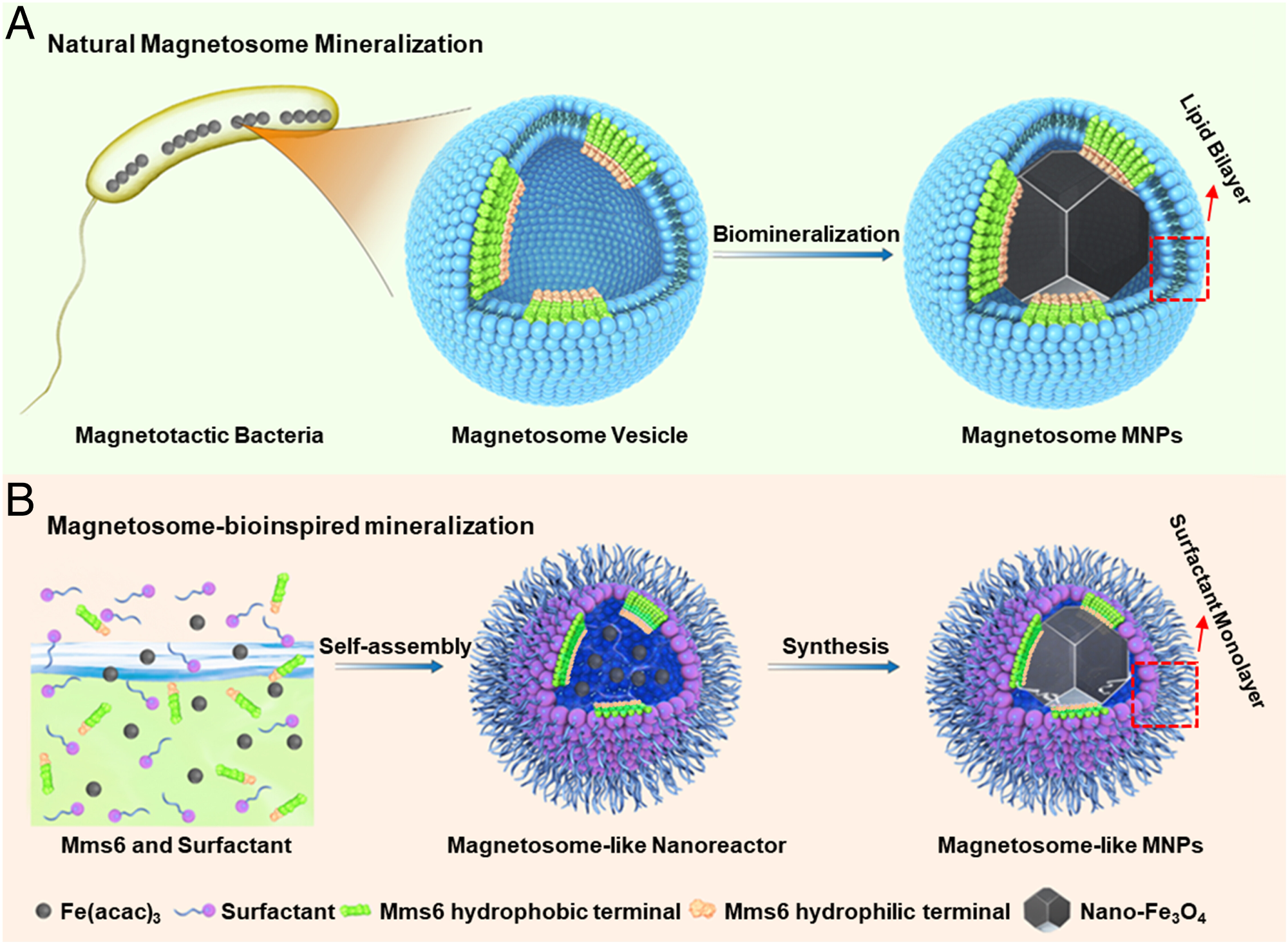
Fig. 1. Nanoscale reaction chambers for biomineralization. (A) Magnetosome from MTB, where the Mms6 protein assembles into a mineralization template on the magnetosome membrane inner surface. The hydrophilic acid–rich region of Mms6 is highlighted in light orange and the N-terminal hydrophobic membrane-associating region in green, respectively; the red dotted frame highlights the lipid bilayer of the magnetosome membrane. (B) Schematic illustration of the synthesis process of magnetosome-like MNPs within the nanoreactor of an Mms6-containing reverse micelle. The red dotted frame highlights the surfactant monolayer in the magnetosome-like MNPs.
Novel Targeted Nanodrug Delivery Technique and the Future of Tumor Therapy
The targeted administration of nanodrugs has emerged as one of the promising approaches to nanodrug therapy and tumor imaging with the continued advancement of nanotechnology. Numerous studies in recent years have revealed that although nanodrugs can reach targeted tumor locations, the average tumor targeting accuracy is less than one percent.
As a result, enriching nanodrugs in tumor tissues is still challenging, mainly to increase their effective penetration efficiency. A magnetic targeting technique adds an external magnetic field to a particular region after intravenous injection, enriching and collecting MNPs as they move through the local blood arteries to lessen the near-miss effect of systemic medication delivery.
Due to the dense extracellular matrix (ECM) and the high interstitial fluid pressure in tumor tissues, MNPs exhibit tissue penetration and low targeting in solid tumor tissues, like other nanodrugs. The following attributes of MNPs must be present for a magnetic targeting system to be effective in overcoming these biological barriers- (i) an adequate saturation magnetization (Ms) and magnetic moment to realize a quick and effective response to an external magnetic field, (ii) superparamagnetic properties to prevent MNP agglomeration, and (iii) small size to enhance penetration ability.
The magnetosome, a unique “organelle” created by biomineralization in MTB, is a naturally occurring MNP of biological origin. It is crucial for MTB magnetic navigation in response to the geomagnetic fields. For AMB-1 MTB, the magnetosome, a nanosized mineralization chamber, creates magnetic nanocrystals with a cubo-octahedral form. The AMB-1 MTB responds quickly to an external magnetic field due to its special magnetic characteristics. Also, the magnetosome is a wonderful option for magnetic targeted nanodrug carriers and magnetic resonance imaging (MRI) operations due to its small size, high Ms, superior stability, and minimal toxicity.
However, in biological fluid and water environments, the high magnetic interaction among natural magnetosome particles leads to precipitation and aggregation, substantially impairing their capacity to penetrate tumor cells. Therefore, creating magnetosome-like MNPs that have the benefits of natural magnetosome MNPs without the disadvantages can be an exciting research topic.
In this study, the authors constructed a bio-inspired nanoreactor by integrating Mms6 proteins into a reverse micelle structure. The obtained MNPs’ magnetic characteristics, morphology, and MR relaxation characteristics were investigated and contrasted with the natural magnetosomes made by AMB-1 MTB.
The tumor penetration was improved by order of magnitude owing to the small size of the magnetosome-like MNPs and their powerful magnetic targeting capacity created by soft ferromagnetism, revealing a beneficial contrast in the tumor area. Thus, the magnetosome-like MNPs appeared extremely intriguing for possible nanodrug applications because of their tumor penetration, magnetic targeting, and MR imaging capabilities.
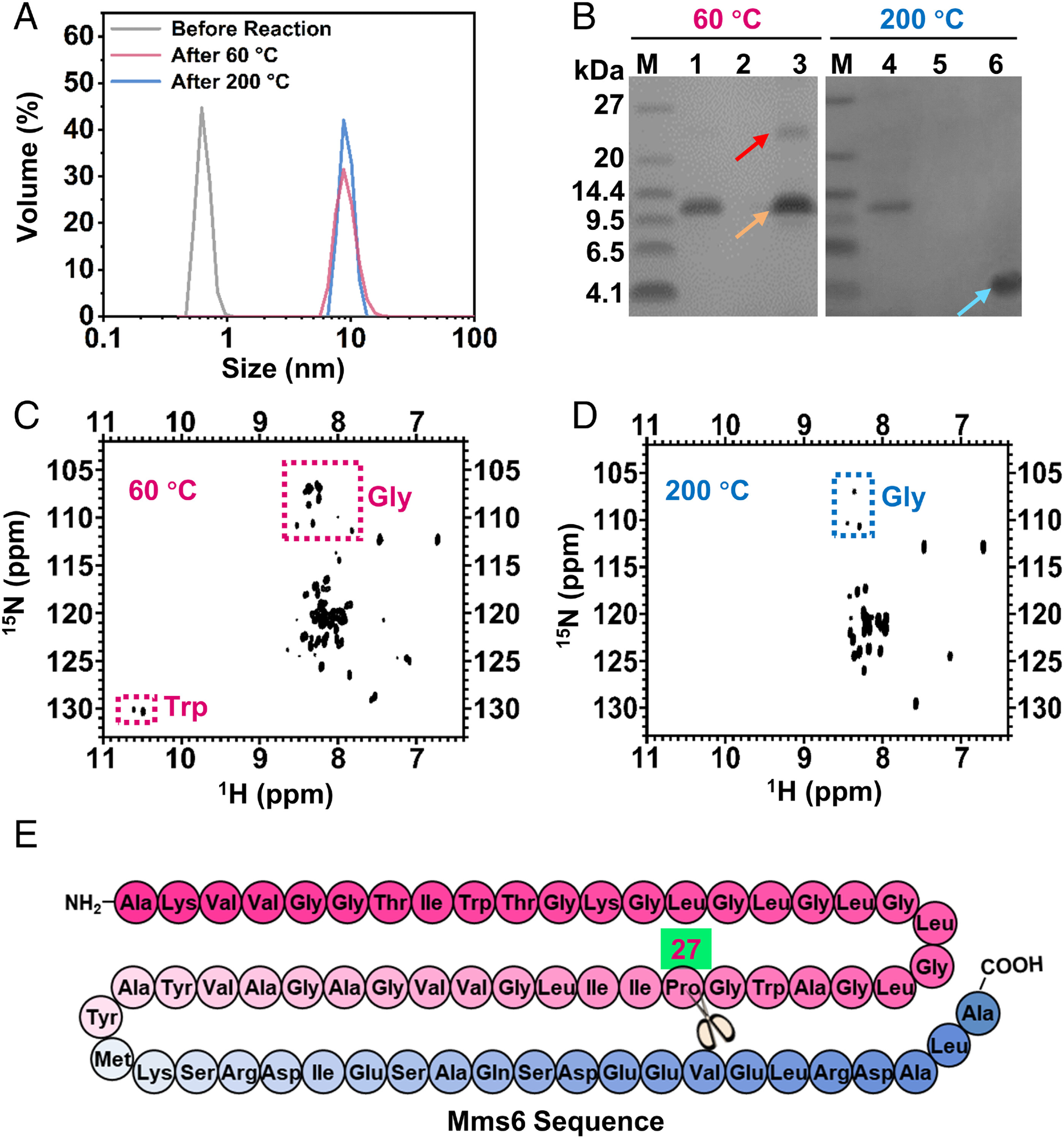
Fig. 2. Construction of the Mms6-containing reverse micelle nanoreactor and characterization of Mms6 during different reaction stages. (A) DLS profiles of the reaction buffer without oleylamine surfactant (gray line), reverse micelle reaction system after reaction at 60 °C for 3 h (red line), and reverse micelle reaction system after reaction at 200 °C for 8 h (blue line). (B) SDS-PAGE analysis of Mms6 after reaction at 60 °C and 200 °C. Lanes 1 and 4, purified Mms6 protein; lanes 2 and 5, supernatant of control reaction without Mms6 participation; lanes 3 and 6, supernatant of reaction at 60 °C (lane 3) and 200 °C (lane 6) with Mms6 participation. Red arrow: Mms6 tetramers; orange arrow: Mms6 dimers; blue arrow: fragments of Mms6 degradation. (C) The 1H,15N-HSQC spectra of Mms6 in the reverse micelle nanoreactor after reaction at 60 °C. Red dotted box: glycine or tryptophan. (D) The 1H,15N-HSQC spectra of Mms6 in the reverse micelle nanoreactor after reaction at 200 °C. Blue dotted box: glycine. (E) Mms 6 sequence with the hydrophilic acid–rich region in blue and the hydrophobic membrane region in pink. The scissors represent the degradation site.
Experimental Set-Up
A nanoreactor that resembled a magnetosome was constructed, and thermal decomposition was utilized to create iron oxide (Fe3O4) MNPs. In a benzyl ether solution with 0.1 percent water, Mms6 protein powder and oleylamine were combined to create the Mms6-containing reverse micelle system. The Mms6 protein was connected to the surfaces of the magnetosome-like MNPs, as shown by the emergence of protein-related FTIR signals. These findings suggested that the reverse micelle system's assembly and the mineralization process required the Mms6 protein.
The tail vein was used to administer the magnetosome-like MNPs to six-week-old male breast tumor model mice. For magnetically focused MNP enrichment, a 0.5 T magnet was placed over the mice's tumor region for 120, 60, and 30 minutes. After being exposed to the magnetic field for 30 minutes, the magnetosome-like MNPs showed a noticeably heightened contrast in the tumor area. Also, the contrast-to-noise ratio (CNR) variation at the tumor rim increased by 132 percent. At the same time, the CNR shift in the tumor interior increased by 110 percent.
Magnetic targeting utilizes magnetic fields to control the distribution of magnetically responsive nanodrug carriers or nanoparticles to minimize the off-target consequences of systemic administration. Iron oxide (FeO) nanoparticles were crucial magnetic targeting nanodrug carriers that were biocompatible and offered a viable nanodrug delivery strategy for cancer treatment. Additionally, due to their large surface area and inherent magnetic and photoelectric capabilities, MNPs could combine various diagnostic procedures, including computed tomography and MRI.
The tumor-targeting evidence showed that magnetosome-like nanoparticles could enter the interior tumor area due to their soft ferromagnetic characteristics and small sizes. Thus, the tumor-targeting efficiency was dramatically increased by about three percent. By contrast, magnetic targeting resulted in a 10 percent reduction in the distribution of magnetosome-like MNPs in the spleen and liver.
These findings demonstrated that magnetosome-like MNPs considerably increased the accuracy of magnetic targeted nanodrug delivery. Due to its remarkable magnetic targeting capability, it was an excellent option for several biomedical applications.
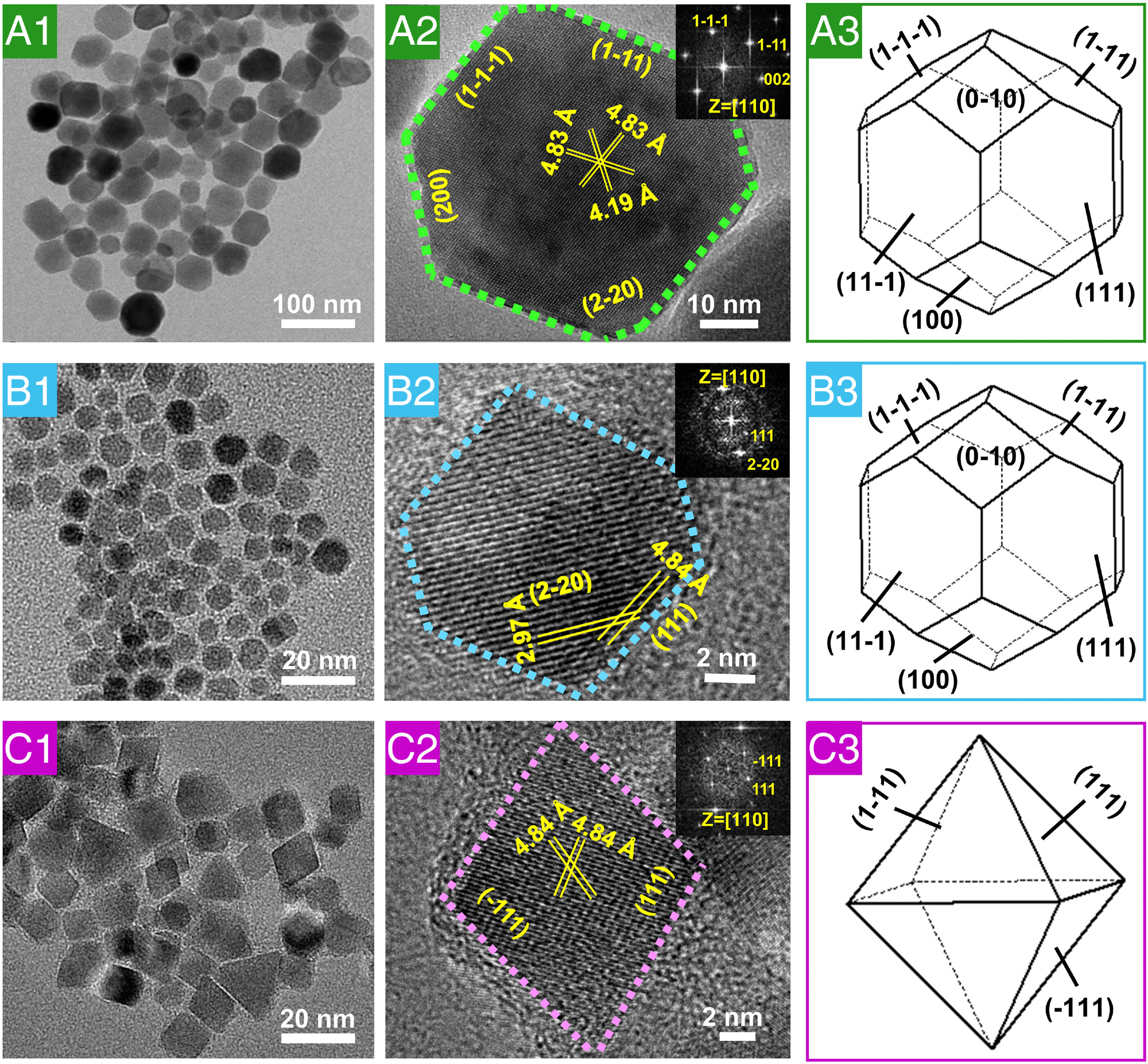
Fig. 3. HRTEM characterization of natural magnetosomes, magnetosome-like MNPs, and magnetic nanocrystals from the control reaction. (A1) and (A2) High-resolution electron micrographs and (A3) three-dimensional morphology of natural magnetosome crystals from AMB-1. (B1) and (B2) High-resolution electron micrographs and (B3) three-dimensional morphology of magnetosome-like MNPs. (C1) and (C2) High-resolution electron micrographs and (C3) three-dimensional morphology of MNPs from the control reaction. The insets in (A2), (B2), and (C2) show the corresponding fast Fourier transform patterns of the crystal structure.
Significance of the Study
In this study, the authors built a magnetosome-like nanoreactor by inserting the amphiphilic Mms6 proteins into the self-assembled reverse micelles, recapitulating the two key elements necessary for MTB biomineralization, including magnetosome regulatory proteins and magnetosome vesicles.
Magnetosome-like MNPs exhibited identical crystalline structures as natural magnetosomes with robust magnetic characteristics that react quickly to external magnetic fields. Additionally, magnetosome-like nanoparticles covered with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine with conjugated methoxyl polyethylene glycol (DSPE-mPEG) showed high good water solubility, monodispersity, and small hydrodynamic dimensions to counteract the drawbacks of natural magnetosome MNPs.
The findings showed that extremely water-soluble magnetosome-like MNPs could offer many additional applications for MR imaging, magnetic hyperthermia, and magnetically guided nanodrug release if customized with the appropriate drugs and target molecules.
Reference
Ma, K et al. (2022). Magnetosome-inspired synthesis of soft ferrimagnetic nanoparticles for magnetic tumor targeting. PNAS. https://www.pnas.org/doi/10.1073/pnas.2211228119
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.