Water-containing layered materials have many potential applications, such as water purification and energy storage. However, the link between interlayer ion configuration and water architecture in high ion retention layered substances is poorly known.
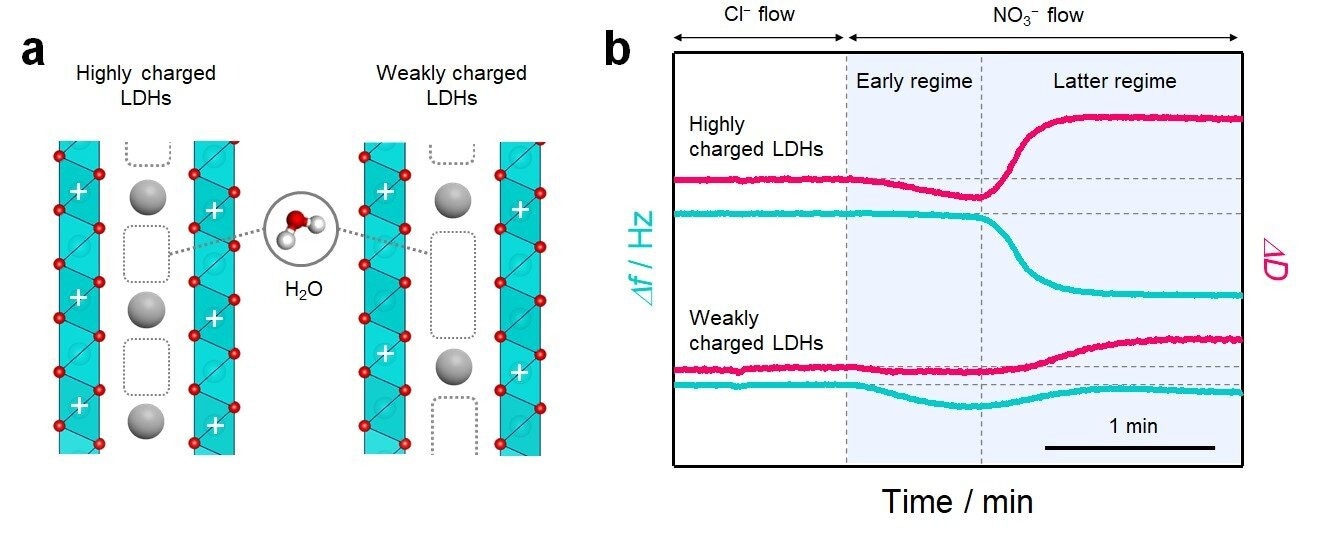
(a) Schematic of the interlayer structure in layered materials with different host charge densities. In the interlayer space, water molecules are incorporated into the pores that are not filled with charge compensating ions for host charge. (b) Quartz crystal microbalance with energy dissipation monitoring (QCM-D) profiles of ion-exchange reaction in LDHs with different host charge densities showing the change in the frequency (Δf) and dissipation (ΔD). Credit: Nature Communications (2022). DOI: 10.1038/s41467-022-34124-9
A recent study published in the journal Nature Communications focuses on this issue by investigating the relationship between water structure and ion storage in layered materials.
Water-Containing Layered Materials
Water-containing layered materials consist of layers stacked on top of each other, with water molecules confined in between the layers. These materials are characterized by their layered structure, composed of positively and negatively charged layers.
The layers are held together by weak forces such as van der Waals forces, and the water molecules are held in place by hydrogen bonds.
Due to their unique properties, water-containing layered materials have found applications in various fields, such as water purification, energy storage, catalysis, and environmental remediation.
These materials are also known as layered double hydroxides (LDHs), composed of positively charged layers made up of metal cations and negatively charged layers made up of hydroxide anions. The layers are stacked together with water molecules or other anions in between the layers, and the distance between the layers can vary depending on the application.
Water-containing layered materials have remarkable ion exchange capacity, which allows them to selectively adsorb specific ions from aqueous solutions. This makes them useful for water purification, as they can remove impurities from water by adsorbing them into the interlayer space. Additionally, their ability to store ions makes them potential candidates for energy storage applications.
Limitations of Water-Containing Layered Materials
Water-containing layered materials, also known as layered double hydroxides (LDHs), have been widely studied due to their unique properties and potential applications in fields. However, despite their potential, several challenges still need to be addressed to realize the full potential of these materials.
One of the main challenges is the lack of understanding of the relationship between the configuration of interlayer ions and water structure in high ion storage layered materials. The capacity to store ions is determined by the properly structured water molecules that surround them under the confinement of the layers.
However, the link between interlayer ion configuration and water structure in high ion retention layered materials is poorly known. This lack of understanding limits the ability to optimize the properties of these materials for specific applications.
Methodology Adopted in this Study
The methodology used in the research article provided combined different techniques to study the relationship between the water structure and ion storage in layered double hydroxides (LDHs). The main technique used to study the water structure was Quartz crystal microbalance with dissipation monitoring (QCM-D).
This technique utilizes a quartz crystal resonator coated with the sample material and measures the changes in the resonant frequency and dissipation of the crystal caused by the adsorption or desorption of molecules on the surface of the crystal. QCM-D was used to monitor the changes in water structure as a function of ion filling density in the interlayer space.
Additionally, multimodal ex-situ experiments were also performed to study the water structure and ion storage in the material. These experiments included X-Ray diffraction, Fourier transform infrared spectroscopy, and thermogravimetric analysis. Theoretical calculations were also performed to understand the relationship between the water structure and ion storage.
Important Developments of the Research
The main conclusion of the research is that the water structure is responsive to the ion filling density in the interlayer region and controls ion storage. According to the research, a 24% drop in filling density triples the nitrate storage capacity.
The researchers also found that decreasing the filling density enables the 2D hydrogen-bond structure in the water around interlayer nitrate ions, leading to high storage capacity.
"Put another way, the water structures are sensitive to how the interlayer ions are structured," said Katsuya Teshima, corresponding author of the study. "And while this ion configuration in many different crystal structures controls how many ions can be stored, such configurations until now had rarely been systematically investigated."
These findings could have implications for future energy storage and water purification technologies. The study showed that by controlling the filling density of ions in the interlayer space, the ion storage capacity of LDHs can be significantly increased.
This could lead to the development of more efficient energy storage devices such as batteries and supercapacitors. Additionally, the ability to control the water structure in LDHs could help to design more effective water purification technologies.
Reference
Sudare, T. et al. (2022). Critical role of water structure around interlayer ions for ion storage in layered double hydroxides. Nature Communications. Available at: https://doi.org/10.1038/s41467-022-34124-9
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.