Researchers from the Indian Institute of Technology Gandhinagar, Sandia National Laboratories, and Lawrence Berkeley National Laboratory worked together to construct 3–4 nm ultrathin nanosheets of a metal hydride that boosts hydrogen storage capacity. The study is published in the journal Small.
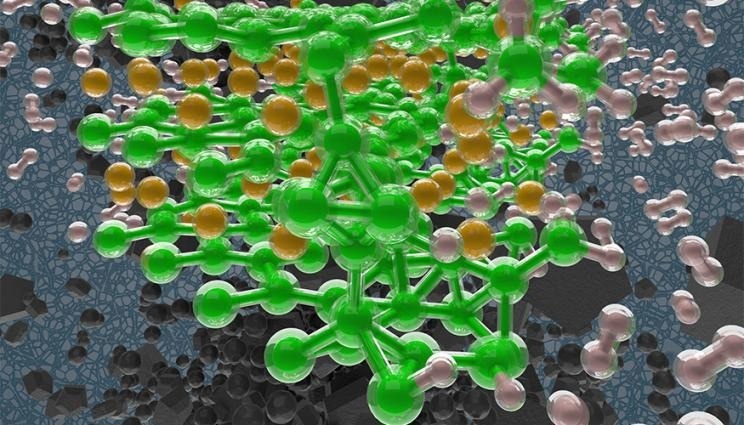
Hydrogen absorption at the surface of magnesium diboride (MgB2) studied with first principles simulations. The background depicts MgB2 crystallites. Image Credit: Liam Krauss/LLNL
Sustainable energy storage systems are required to counteract the erratic nature of renewable energy sources. Technologies based on hydrogen provide potential long-term approaches to lowering greenhouse gas emissions. With hydrogen having the highest energy density of any fuel, it is thought to be a practical option for marine, air, and land vehicles.
However, in terms of volumetric energy density, hydrocarbon fuel sources surpass compressed hydrogen gas, spurring the creation of substitute, more energy-dense material-based storage techniques.
Although they have a large absolute storage capacity for hydrogen, complex metal hydrides are a class of hydrogen storage materials that have the potential to be exposed to extremely high pressures and temperatures.
The scientists overcame this difficulty by nano-sizing, which enhances the surface area available for hydrogen reactions and reduces the necessary depth of hydrogenation. Magnesium diboride (MgB2) has been studied at the nanoscale in previous research, including work by LLNL. However, the material in that study was not as thin and ended up clustering.
The material developed in this most recent partnership was produced via solvent-free mechanical exfoliation in zirconia, resulting in a material that is just 11–12 atomic layers thick and can hydrogenate to a capacity of around 50 times that of the bulk material.
This 50-fold increase in hydrogenation neatly correlates with a 50-fold rise in surface-to-volume ratio, indicating that both the bulk and nanosheet material hydrogenate roughly the first two layers, a behavior that is independent of particle size. This is equivalent to a third of MgB2’s maximal hydrogen capacity for two layers on either side of the 11–12-layer nanomaterial.
The stability of the boron layer is driven by charge transfer from the magnesium layer to the boron layer in MgB2, which is composed of alternating layers of magnesium and boron.
According to LLNL simulations, the material’s insufficient magnesium coverage promotes a surface structure with islands of fully covered magnesium and other regions with less stable disordered surface boron layers.
Calculations demonstrate how magnesium coverage on MgB2 changes when it hydrogenates, building on previous research on the disordering of surface boron layers.
These results show how a reactive MgB2 surface with exposed boron may become more stable as it hydrogenates because the magnesium coverage increases. By this mechanism the hydrogenation slows and halts for moderate hydrogenation conditions.
Keith Ray, Study Author and Physicist, Lawrence Livermore National Laboratory
He added, “Further nano-sizing or a novel chemical modification to delay or disrupt the increase in surface magnesium may further increase MgB2 performance as a hydrogen storage material.”
Maxwell Marple, Sichi Li, and Brandon Wood are other LLNL authors.
Hydrogen Storage Materials Advanced Research Consortium (HyMARC) in the Department of Energy, Office of Energy Efficiency and Renewable Energy, Hydrogen & Fuel Cell Technologies Office provided funding for the study.
Journal Reference
Gunda, H., et al. (2023) Hydrogen Storage in Partially Exfoliated Magnesium Diboride Multilayers. Small. doi:10.1002/smll.202205487.