The first selective treatment to stop allergic responses has been created by Northwestern University researchers. These reactions can range in severity from watery eyes and itchy hives to breathing difficulties and even death.
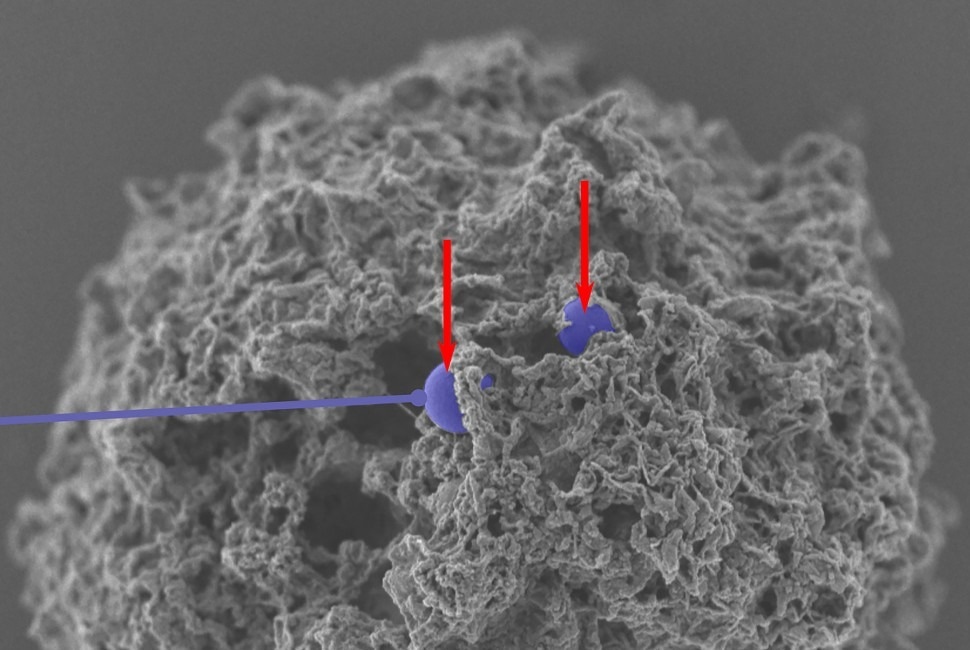 A scanning electron microscope image of nanoparticles (colorized in purple) that have successfully entered a mast cell. Image Credit: Northwestern University
A scanning electron microscope image of nanoparticles (colorized in purple) that have successfully entered a mast cell. Image Credit: Northwestern University
The foundation of the new treatment comes from nanoparticles that were decorated with antibodies and an allergen that matches the patient's particular sensitivity. For example, if a person has a peanut allergy, the nanoparticle contains a peanut protein. Doing so enabled these nanoparticles to inhibit the function of mast cells, a type of immune cell which are in charge of triggering allergic reactions.
Using this two-step process, the allergen interacts with the particular mast cells that cause the allergy, and only those cells are subsequently inhibited by the antibodies. The treatment can selectively avoid particular allergies without completely weakening the immune system due to its highly focused approach.
The treatment showed 100% efficacy in avoiding allergic reactions in a mouse trial with few adverse effects.
The study was published in Nature Nanotechnology on January 16th, 2023 and represents the first instance of mast cell inhibition using nanotherapy.
Currently, there are no methods available to specifically target mast cells. All we have are medications like antihistamines to treat symptoms, and those don’t prevent allergies. They counteract effects of histamines after the mast cells already have been activated. If we had a way to inactivate the mast cells that respond to specific allergens, then we could stop dangerous immune responses in severe situations like anaphylaxis as well as less serious responses like seasonal allergies.
Evan A. Scott, Study Lead, Professor, Biomedical Engineering, Northwestern University
Dr. Bruce Bochner, an allergy expert and study co-author, added, “The biggest unmet need is in anaphylaxis, which can be life-threatening. Certain forms of oral immunotherapy might be helpful in some cases, but we currently don’t have any FDA-approved treatment options that consistently prevent such reactions other than avoiding the offending food or agent. Otherwise, treatments like epinephrine are given to treat severe reactions — not prevent them. Wouldn’t it be great if there was a safe and effective treatment for food allergy that consistently made it possible to reintroduce a food into the diet that you used to have to strictly avoid?”
In addition to being a member of the Chemistry of Life Processes Institute, the International Institute for Nanotechnology, and the Simpson Querrey Institute for BioNanotechnology, Scott holds the position of Kay Davis Professor of Biomedical Engineering at Northwestern’s McCormick School of Engineering. Bochner teaches allergy and immunology at Northwestern University Feinberg School of Medicine as the Samuel M. Feinberg Emeritus Professor of Medicine.
Fanfan Du, a postdoctoral fellow in Scott’s lab, is the study’s first author. He collaborated closely with Yang Li, a Ph.D. candidate in Scott’s lab, and Clayton Rische, a Ph.D. candidate who was mentored by both Bochner and Scott.
Tricky Target
Mast cells are found in almost every tissue in the human body, although they are most well-known for being the main cause of allergic reactions. However, they also carry out a number of other crucial functions, including controlling blood flow and combating parasites. Thus, eradicating mast cells completely in an attempt to stop allergic reactions can harm other beneficial, healthy reactions.
Dr. Bochner added, “Although some drugs are under development, there are currently no FDA-approved drugs that inhibit or eliminate, mast cells. This has been difficult mainly because drugs that can affect mast cell activation or survival also target cells other than mast cells, and thus tend to have unwanted side effects due to influences on other cells.”
Bochner’s previous study revealed Siglec-6, a distinct inhibitory receptor that is primarily and exclusively present in mast cells. To avoid allergies, researchers could specifically block mast cells by targeting that receptor with an antibody. However, merely injecting this antibody was insufficient.
“It was difficult to get a high-enough concentration of the antibody to have an effect. We wondered if we could enhance this concentration using a nanoparticle. If we could pack a high density of antibodies onto a nanoparticle, then we could make it practical for use,” Scott further stated.
Sticking Antibodies onto a Particle
Another obstacle Scott and his colleagues had to face was packing the antibodies onto a nanoparticle. Proteins (like antibodies) usually need to make a chemical connection with the nanoparticle for them to adhere to it. This connection unfolds or denatures the protein, hence altering its biological function. Scott used a nanoparticle he had previously created in his lab to get around this problem.
Scott’s recently discovered nanoparticle has a dynamic polymer chain that can autonomously change its orientation when exposed to different solvents and proteins, in contrast to more conventional nanoparticles that have fixed surfaces. The chains align themselves in liquid solutions to provide advantageous electrostatic interactions with the molecules of water.
However, upon contact with the surface of a nanoparticle, a particular type of small polymer chain at the interface reverses their orientations, allowing the protein to be securely attached without forming a covalent connection. Additionally, Scott’s group discovered that the stable association depended on water-repelling pockets on protein surfaces.
Proteins usually denature when they attach to surfaces, losing their biological function. The ability of Scott’s nanoparticles to stably bind enzymes and antibodies while preserving their three-dimensional structure and biological activities makes them special. This indicates that even after adhering to the surfaces of the nanoparticles, the anti-Siglec-6 antibodies retained their potent affinity for the mast cell receptors.
Scott noted, “This is a uniquely dynamic surface. Instead of a standard stable surface, it can switch its surface chemistry. It is made of tiny polymer chains of compounds, which can flip their orientation to maximize favorable interactions with both water and proteins as necessary.”
Nearly all of the antibodies bound to the nanoparticles without losing their capacity to bind to their intended targets when Scott’s team combined the nanoparticles with the antibodies. This led to the development of a nanoparticle-based treatment that targets mast cells by using surfaces that have highly controlled concentrations of numerous distinct antibodies packed in tightly.
Selective Shut Down
An individual must have mast cells that recognize and express antibodies, more precisely immunoglobulin E (IgE) antibodies, against the allergen in question for them to develop an allergy. This makes it feasible for the mast cells to identify the same allergen again and respond to it.
Scott further stated, “If you have a peanut allergy and have had a response to peanuts in the past, then your immune cells made IgE antibodies against peanut proteins, and the mast cells collected them. Now, they are waiting for you to eat another peanut. When you do, they can respond within minutes, and if the response is strong enough, it can result in anaphylaxis.”
The researchers created a therapy that exclusively activates mast cells that have IgE antibodies against a given allergen to specifically target mast cells to react to that allergen. The nanoparticle binds to the mast cells’ IgE antibodies using a protein allergen, and then it utilizes an antibody to bind to the Siglec-6 receptor, which inhibits the mast cells’ capacity to respond.
Additionally, the nanoparticle cannot attach to other cell types since only mast cells have Siglec-6 receptors, which effectively restricts adverse effects.
“You can use any allergen that you want, and you will selectively shut down the response to that allergen. The allergen would normally activate the mast cell. But at the same time the allergen binds, the antibody on the nanoparticle also engages the inhibitory Siglec-6 receptor. Given these two contradictory signals, the mast cell decides that it shouldn’t activate and should leave that allergen alone,” Scott stated.
He continued, “It selectively stops a response to a specific allergen. The beauty of this approach is that it does not require killing or eliminating all the mast cells. And, from a safety standpoint, if the nanoparticle accidentally attaches to the wrong cell type, that cell just won’t respond.”
Preventing Anaphylaxis in Mice
The researchers successfully tested their treatment in cellular cultures using mast cells taken from human tissue before transferring them to a humanized mouse model. Because mice’s mast cells lack the Siglec-6 receptor, Bochner’s group created a mouse model in which the tissues included human mast cells. Simultaneously, the researchers administered the nanotherapy and exposed the mice to an allergen.
None of the mice had anaphylactic shock, and they all lived.
Scott added, “The simplest way to monitor an allergic response is to track changes in body temperature. We saw no changes in temperature. There was no response. Also, the mice remained healthy and did not display any outward signs of an allergic reaction.”
“Mouse mast cells do not have Siglec-6 on their surface like in humans, but we got as close as we could for now to actual human studies by testing these nanoparticles in special mice that had human mast cells in their tissues. We were able to show that these humanized mice were protected from anaphylaxis,” Bochner added.
The researchers intend to investigate the potential of their nanotherapy for the treatment of additional mast cell-related conditions, such as mastocytosis, a rare kind of mast cell cancer. To selectively destroy mast cells during mastocytosis without harming other cell types, they are also looking at ways to pack drugs inside nanoparticles.
The National Institute of Allergy and Infectious Disease (grant number R21AI159586) and the National Institute of Biomedical Imaging and Bioengineering (grant number 1R01EB030629-01A1) provided funding for this study.
Journal Reference:
Du, F., et. al. (2023) Controlled adsorption of multiple bioactive proteins enables targeted mast cell nanotherapy. Nature Nanotechnology. doi:10.1038/s41565-023-01584-z.