A new study published in the journal molecules has aimed to investigate the immunotherapy effect and potential mechanism of recombinant Amb a 1-loaded poly(DL-lactide-co-glycolide)-polyethylene glycol (PLGA-PEG) nanoparticles for reducing allergic conjunctivitis.

Study: Effects of rAmb a 1-Loaded PLGA-PEG Nanoparticles in a Murine Model of Allergic Conjunctivitis. Image Credit: Elizaveta Galitckaia
Allergic conjunctivitis is caused by Ambrosia artemisiifolia (Amb a), which consists of numerous allergens.
Allergic Conjunctivitis – What is it?
Allergic conjunctivitis (AC) consists of the inflammation of the conjunctiva, comprising the membrane covering the white part of the eye; this occurs as a result of an allergy, with the most common cause being hay fever. AC describes a group of ocular conditions which are associated with a hypersensitivity to immunoglobulin E (IgE).
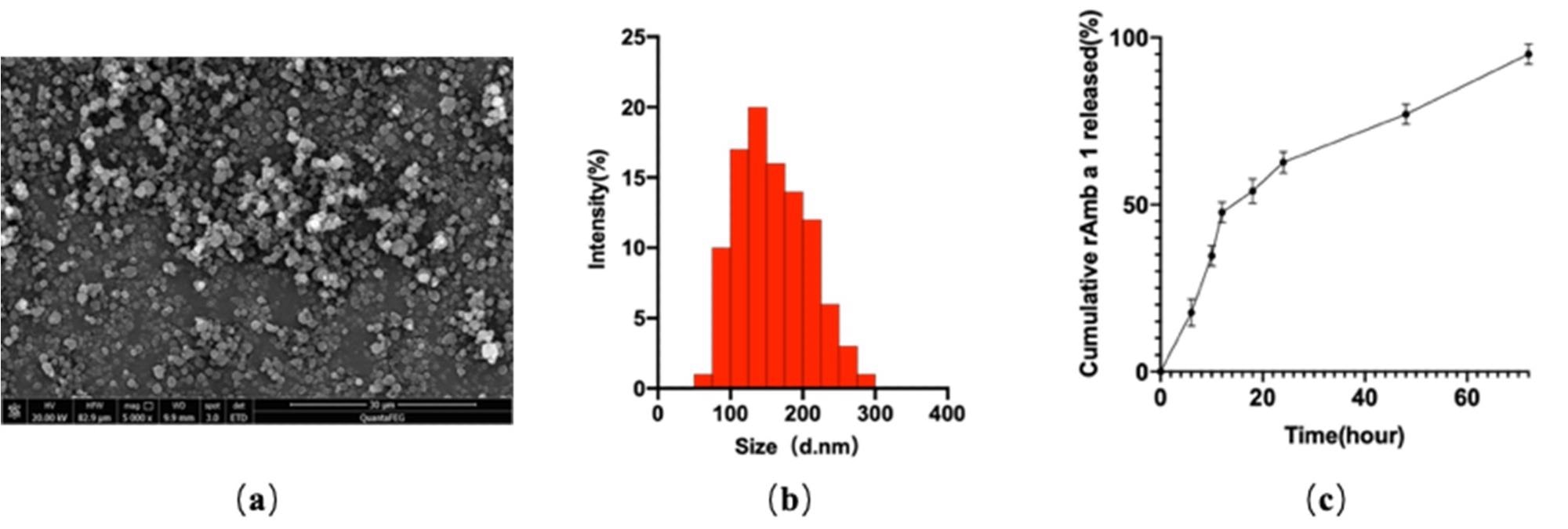
Figure 1. (a) Field emission scanning electron microscopic image; (b) dynamic light-scattering spectra; (c) in vitro cumulative protein release of rAmb a 1-loaded PLGA-PEG nanoparticles. Abbreviations: PLGA-PEG, poly(DL-lactide-co-glycolide)-polyethylene glycol; rAmb a 1, recombinant Amb a1. © Cao, H., Liu, L., Wang, J., Gong, M., Yuan, R., Lu, J., Xiao, X. and Liu, X., (2022)
Immunoglobulin E hypersensitivity reactions can be caused or stimulated by various factors, as described by the National Institute for Health and Care Excellence (NICE), including, seasonal allergic conjunctivitis, which is dependent on seasonal changes which stimulate allergies, due to allergens such as trees and pollen, and Perennial allergic conjunctivitis, consisting of non-seasonal environmental allergens typically found at home including dust mites and mold spores.
Approximately up to 40 percent of the population have experiences related to allergic conjunctivitis, the most common type of ocular allergic diseases.
Simple ocular allergies can affect between 10-30 percent of the general population; however, these conditions generally have a small population that seek medical assistance.
Novel Treatments for Allergic Conjunctivitis
Allergen-specific immunotherapy (AIT) is currently the only cause-specific therapy available for allergic diseases, with this approach aiming to stimulate allergic patients utilizing exposure to a low dose of allergens and increasing it to a high dose with the final expectation being a lack of response towards the specific stimulating allergen.
This method of building immune tolerance can be successful, however, clinical vaccine products which utilize crude extracts of allergens can lead to adverse reactions due to significant differences in batches and can result in treatment failure.
The current challenges that are faced within AIT vaccines are aimed to be solved with this novel research. This study includes the use of PLGA (polylactic acid–glycolic acid), which is a non-toxic, biodegradable polymer material approved by the FDA (Food and Drug Administration) and can be used as a support material for the application of clinical drugs.
Additionally, the combination of PLGA and another polymer material approved by the FDA, known as PEG (Polyethylene Glycol), and creating a duo of PLGA-PEG, has the potential to be effective for a novel AIT vaccine.
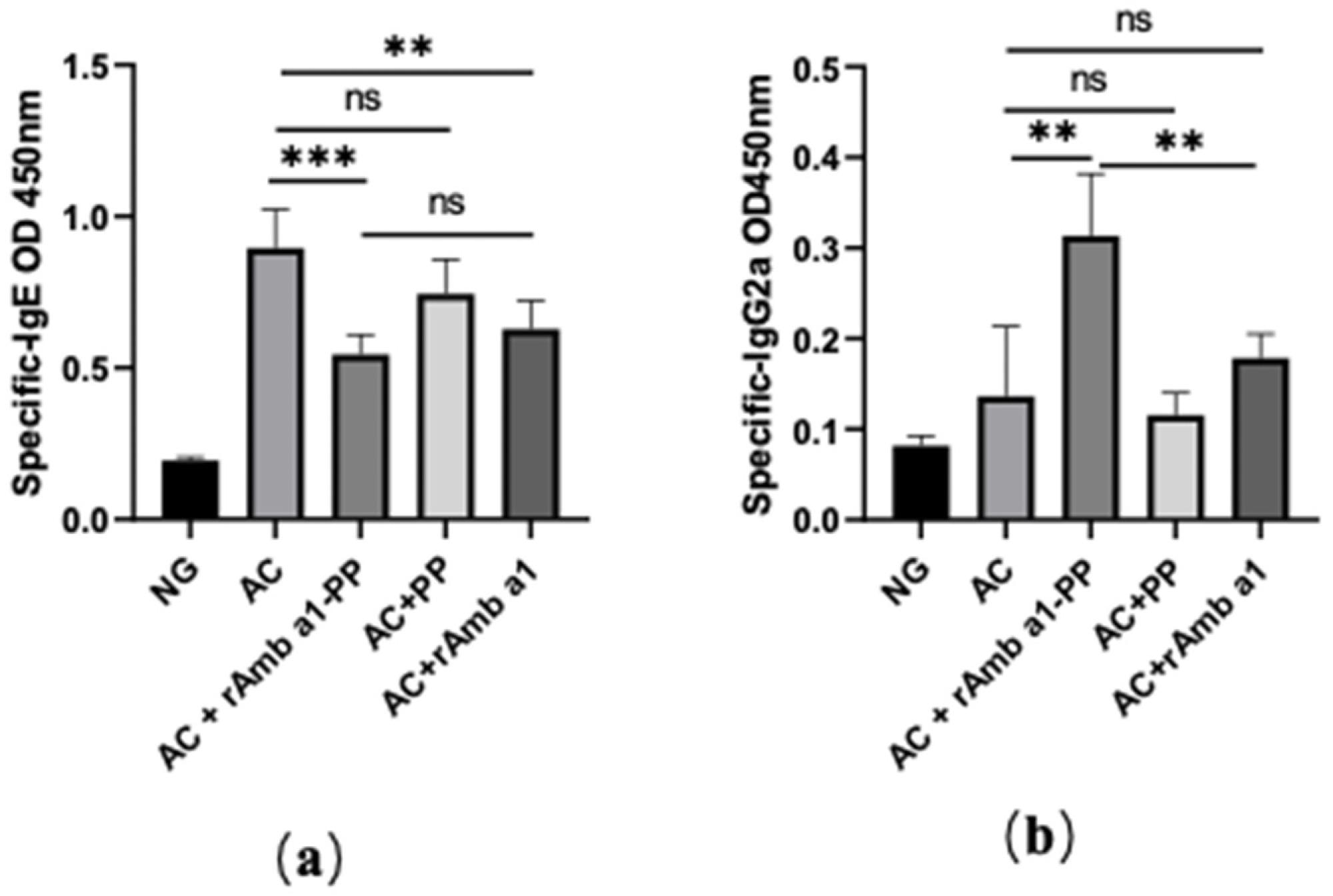
Figure 2. Serum-specific IgE (a) and IgG2a (b) in mice in each group. The data are shown as mean ± SD from five individual mice (*** p < 0.001, ** p < 0.01, ns: no significant difference). Abbreviations: NG, naive group; AC, allergic conjunctivitis group; AC + rAmb a 1-PP, allergic conjunctivitis + rAmb a 1-PLGA-PEG treatment group; AC + PP, allergic conjunctivitis + PLGA-PEG treatment group; AC + rAmb a 1, allergic conjunctivitis + rAmb a 1 treatment group. © Cao, H., Liu, L., Wang, J., Gong, M., Yuan, R., Lu, J., Xiao, X. and Liu, X., (2022)
PEG consists of beneficial characteristics which has raised its potential for use, including having a long internal circulation, affinity for dissolving in water and organic solvents, whilst also supplementing the hydrophobicity of PLGA.
The researchers of this study have utilized nanoparticles comprising of PEG within PLGA-carrier materials which aim to provide a synergy between these two polymers. This would aim to combine a drug delivery carrier within a controlled-release mode with biodegradability, high safety, and stability advantages, as well as prolonged immunity.
The team utilized a recombinant Amb a 1-loaded PLGA-PEG nanoparticles in order to treat allergic conjunctivitis as a result of a crude extract of Ambrosia artemisiifolia.
Their results consisted of finding the combination of PLGA-PEG as a nanodrug carrier to be effective, and with the recombinant allergen protein encapsulated within these nanocarriers and released into patients with allergies providing allergen specific immune tolerance.
The study utilized a mouse allergic conjunctivitis model and had a release rate of 72 hours for the allergens within the nanoparticles – a slow release that enabled increased interaction time between the antigen and the immune cells.
This factor may have contributed to the proof-of-concept success of utilizing allergen loaded PLGA-PEG nanoparticles for hypersensitivity and allergy treatment.
Future Outlook on Allergy Treatments
This research is significant for medicine and those burdened with having hypersensitivity towards allergens as it could potentially provide a nanovaccine intervention against Type I allergies.
With this injection resulting in the inhibition of an allergic reaction against Ambrosia artemisiifolia and the specific decrease of associated IgE serum levels, the translational implication for this research could be revolutionary.
Suppressing allergic responses through this manner and being able to modify it with specific allergens could assist in reducing the population experiencing allergies. This would enable a higher quality of life and expand the treatments available for allergies and associated hypersensitivity towards ubiquitous allergens.
Continue reading: Developing Lactose-Free Milk with Graphene Oxide Based Nanofiltration Membranes.
Reference
Cao, H., Liu, L., Wang, J., Gong, M., Yuan, R., Lu, J., Xiao, X. and Liu, X., (2022) Effects of rAmb a 1-Loaded PLGA-PEG Nanoparticles in a Murine Model of Allergic Conjunctivitis. Molecules, 27(3), p.598. Available at: https://www.mdpi.com/1420-3049/27/3/598/htm
Further Reading
Baab S, Le PH, Kinzer EE. Allergic Conjunctivitis. (2021) In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448118/
Cks.nice.org.uk. (2022) Conjunctivitis - allergic | Health topics A to Z | CKS | NICE. [online] Available at: https://cks.nice.org.uk/topics/conjunctivitis-allergic/
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.