Reviewed by Lexie CornerApr 1 2025
Researchers from Pennsylvania State University have developed a new method for effectively modeling seed particles composed of 100–200 atoms using a supercomputer. They found that the temperature and solvent composition had unexpected effects on the shapes of these microscopic particles. Interestingly, adding or removing just one atom can sometimes lead to a significant change in the particle's shape.
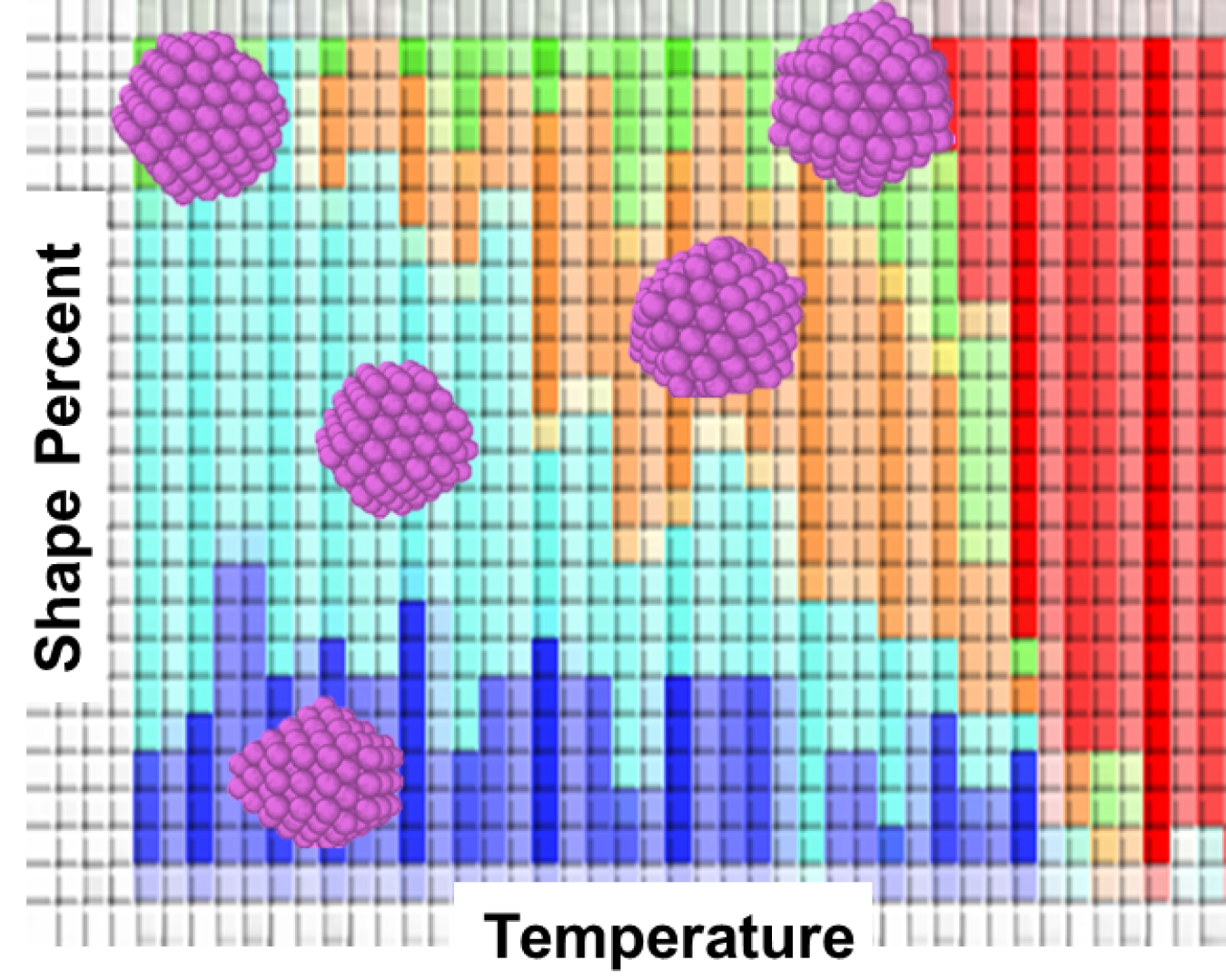 Simulated predicted shape distribution for magic-sized silver clusters (purple insets) assembled at different solvent temperatures. These predictions can guide experiments to target seeds for crystal growth. Image Credit: Kristen Fichthorn, The Pennsylvania State University
Simulated predicted shape distribution for magic-sized silver clusters (purple insets) assembled at different solvent temperatures. These predictions can guide experiments to target seeds for crystal growth. Image Credit: Kristen Fichthorn, The Pennsylvania State University
The Science
Scientists have long understood that the temperature and solvent selection during nanoparticle growth affect the shape of the particles. However, it has been difficult to precisely measure the tiny seed particles that form initially and direct the development of the final nanoparticle shapes.
The Impact
Wearable electronics, solar cells, transparent conducting films, electromagnetic shielding, and catalysis are some of the applications for metal nanoparticles. To achieve optimal performance in these technologies, it is essential to control the shape of the nanoparticles.
The development of metal nanoparticles with regulated size and shape presents a significant challenge for scientists. This study represents a notable advancement in the modeling of seed particle shapes.
The researchers have shown how solvents and temperature influence the shape of nanoparticles. These models indicate promising approaches for producing nanoparticles with the desired sizes and shapes.
Summary
A significant challenge in materials synthesis is growing metal nanocrystals with controlled shapes and sizes. These nanocrystals are typically formed in the solution phase when a metal salt undergoes reduction by a solvent or additives.
Metal atoms and/or ions then cluster together to form nuclei, which evolve into seeds with sizes on the nanometer scale. These seeds continue to grow into the final shapes of the nanocrystals, and controlling the seed-crystal shapes is crucial, as the final nanocrystal shapes are determined by the seed shapes.
Researchers used two computational methods—partial replica exchange MD and parallel-tempering MD—to estimate the most likely shapes of silver nanocrystals in a vacuum, ethylene glycol (EG) solvent, and EG solvent with a growth-directing chemical (polyvinylpyrrolidone). These studies revealed that at specific critical sizes, adding or removing a single atom can cause a significant change in the shape of nanocrystals.
These critical sizes may represent turning points along the nanocrystal growth trajectory. Identifying these critical sizes is essential to developing processing methods that will yield a specific nanocrystal shape.
Temperature also plays a crucial role in the growth trajectory of nanocrystals. At certain critical sizes, one shape is more likely to occur at low temperatures, while another shape is more likely to occur at high temperatures.
Journal Reference:
Yan, T., et al. (2023) Minimum Free-Energy Shapes of Ag Nanocrystals: Vacuum vs Solution. ACS Nano. doi.org/10.1021/acsnano.3c06395.