Guilin University of Technology researchers have created a more flexible, affordable, safer, and high-performing battery option for wearable technology. On June 3rd, 2024, a study outlining the “recipe” for their novel battery type was published in Nano Research Energy.
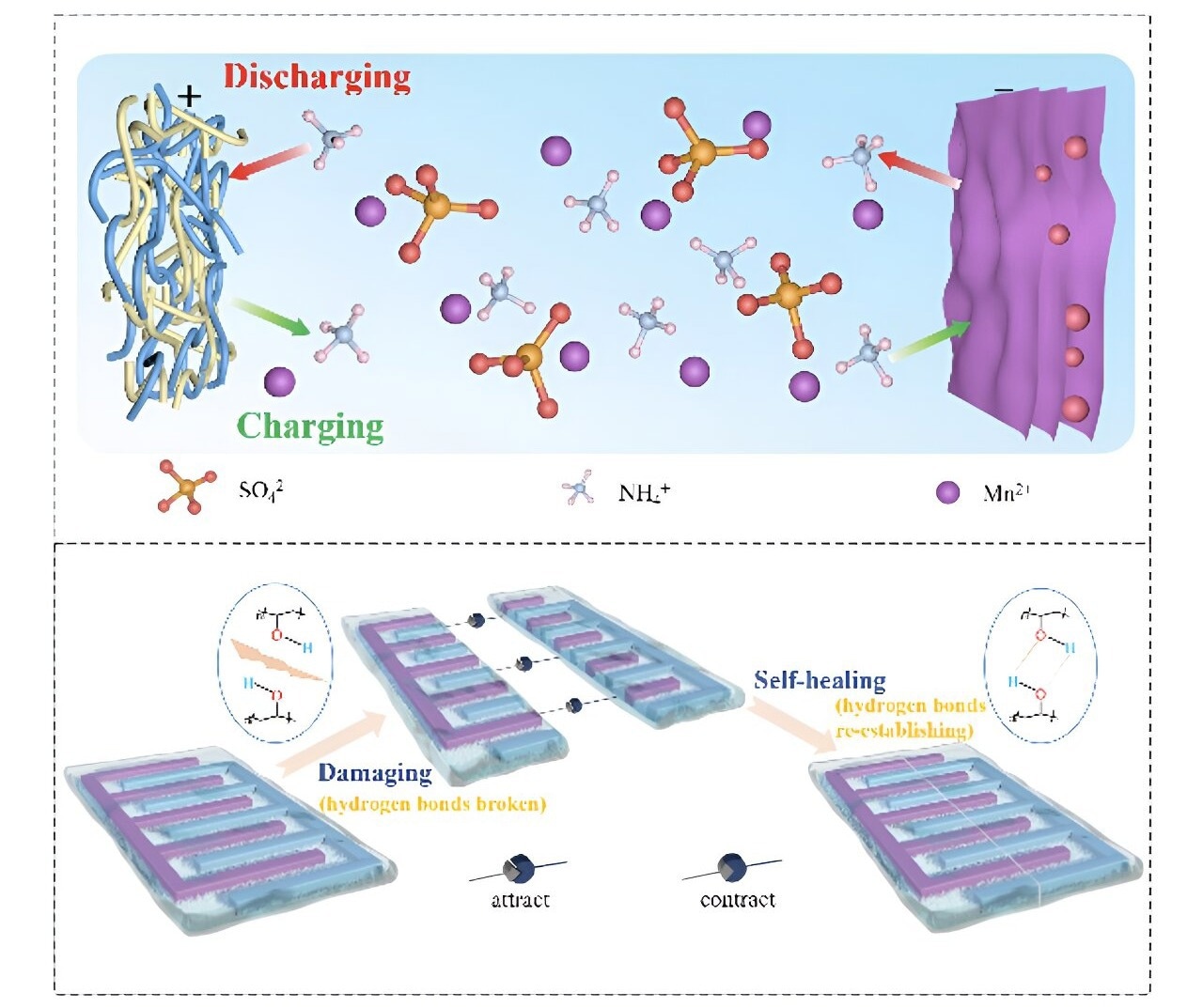 The discharging and charging mechanism of the battery (top), and its self-healing mechanism (bottom). Image Credit: Nano Research Energy, Tsinghua University
The discharging and charging mechanism of the battery (top), and its self-healing mechanism (bottom). Image Credit: Nano Research Energy, Tsinghua University
Fitness trackers, smart watches, virtual reality headsets, smart clothes, and implants—wearable electronic devices are everywhere. However, these devices require more flexible and miniaturized energy storage systems to enhance comfort, reliability, and durability, as current options are often bulky, heavy, and fragile. Additionally, any advancements must not compromise security.
This is why the creation of “micro” flexible energy storage devices, or MFESDs, has been the subject of much battery research in recent years. Water-based microbatteries have been studied with various configurations and electrochemical foundations; among them, they have some unique benefits.
Aqueous batteries, which employ a water-based solution as the electrolyte (the medium that permits ion transport in the battery and so creates an electric circuit), are nothing new. Their existence dates back to the late nineteenth century.
However, their energy density—the amount of energy held in the battery per unit of volume—is insufficient for usage in electric vehicles since they would take up too much room. Lithium-ion batteries are significantly more suited for such applications.
At the same time, aqueous batteries are far less flammable and safer than lithium-ion batteries. They are also significantly cheaper. Because of their superior safety and low cost, aqueous options are rapidly being considered one of the best alternatives for MFESD. These are known as aqueous micro batteries, or simply AMBs.
Up till now, sadly, AMBs have not lived up to their potential, to be able to be used in a wearable device, they need to withstand a certain degree of real-world bending and twisting. But most of those explored so far fail in the face of such stress.
Ke Niu, Study Lead Researcher and Materials Scientist, Guangxi Key Laboratory of Optical and Electronic Materials and Devices, Guilin University of Technology
To circumvent this, any AMB fractures or failure points would need to be able to mend themselves after such stress. Sadly, metallic compounds have typically been used as the charge carriers in the battery’s electric circuit by the self-healing AMBs produced up to this point.
This has the undesired side effect of causing a strong interaction between the metal ions and the materials used to make the electrodes (the positive and negative electrical conductors of the battery). This, in turn, limits the battery’s response rate (the pace at which the electrochemical processes at its heart occur), severely restricting performance.
So we started investigating the possibility of non-metallic charge carriers, as these would not suffer from the same difficulties from interaction with the electrodes.
Junjie Shi, Study Lead Researcher, School of Physics and Center for Nanoscale Characterization & Devices, Huazhong University of Science and Technology
The research team identified ammonium ions, derived from readily available ammonium salts, as the optimal charge carriers for their new battery technology. These ions are significantly less corrosive than other alternatives and offer a wide electrochemical stability window.
"But ammonium ions are not the only ingredient in the recipe needed to make our batteries self-healing," said Long Zhang, the third leading research team member, also at CNCD.
To achieve self-healing properties, the team integrated the ammonium salts into a hydrogel—a polymer material capable of absorbing and retaining large amounts of water without compromising its structure. This characteristic grants hydrogels remarkable flexibility, which is essential for self-healing. While gelatin is a well-known hydrogel, the researchers chose polyvinyl alcohol hydrogel (PVA) for its superior strength and affordability.
For optimal compatibility with the ammonium electrolyte, titanium carbide—a "2D" nanomaterial with a single layer of atoms—was selected for the anode material due to its excellent conductivity. For the cathode, manganese dioxide, a common material in dry cell batteries, was embedded in a carbon nanotube matrix to enhance conductivity.
Testing of the prototype self-healing battery demonstrated excellent energy density, power density, cycle life, flexibility, and self-healing capability, even after ten self-healing cycles.
The team now aims to refine and optimize their prototype further for commercial production.
Journal Reference:
Niu, K., et al. (2024) A self-healing aqueous ammonium-ion micro batteries based on PVA-NH 4Cl hydrogel electrolyte and MXene-integrated perylene anode. Nano Research Energy. doi:10.26599/nre.2024.9120127