Reviewed by Lexie CornerJul 25 2024
Researchers from Nanjing University have developed a sustainable method for producing acetic acid, a key ingredient in numerous industries, including food, medicine, and agriculture. Compared to traditional methods, the new process significantly reduces energy consumption and hazardous waste generation. The study detailing this breakthrough is published in Carbon Future.
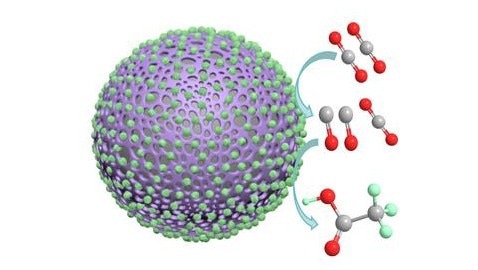 This image shows a polyaniline catalyst coated in cobalt oxide nanoparticles and demonstrates how the catalyst facilitates the conversion of carbon dioxide to carbon monoxide to acetate. Image Credit: Carbon Future, Tsinghua University Press
This image shows a polyaniline catalyst coated in cobalt oxide nanoparticles and demonstrates how the catalyst facilitates the conversion of carbon dioxide to carbon monoxide to acetate. Image Credit: Carbon Future, Tsinghua University Press
The research details a novel method for producing acetate through carbon dioxide electroreduction using a polyaniline catalyst and cobalt oxide nanoparticles.
The polyaniline catalyst with cobalt oxide nanoparticles has two components - polyaniline as a continuous material and cobalt oxide as nanoparticles dispersed on the polyaniline. This cooperative structure makes a highly selective catalyst that can produce acetate during carbon dioxide electroreduction. Cobalt oxide is in charge to produce carbon monoxide intermediate and then pass them to polyaniline, where acetate is formed by electroreduction.
Liwen Wang, Professor, School of Chemistry and Chemical Engineering, Nanjing University
Polyaniline is a conducting polymer that has been proven to be a highly selective catalyst used in the production of other carbon products. This work examines the function of polyaniline and the mechanism of carbon dioxide electroreduction across the polyaniline surface. A greater concentration of carbon monoxide on the polyaniline improves carbon-to-carbon coupling on the catalyst surface. The addition of cobalt oxide nanoparticles as an additional catalyst produces a highly acetate-selective tandem reaction.
This configuration facilitates a higher local carbon monoxide concentration over polyaniline and enhances carbon-to-carbon coupling. The non-metallic polyaniline material can provide excellent performance in electrocatalysts.
Liwen Wang, Professor, School of Chemistry and Chemical Engineering, Nanjing University
Wang continued by describing the way the cobalt oxide nanoparticles and the polyaniline material worked together.
Wang continued, “The polyaniline provides available active sites for increasing the carbon-to-carbon coupling, while the cobalt oxide nanoparticles offer a large number of carbon monoxide intermediates.”
Researchers also prepared two control samples: a polyaniline catalyst without cobalt oxide and a cobalt oxide catalyst; these were used to gauge the performance of the polyaniline/cobalt oxide catalyst. The cobalt oxide nanoparticle deposits were uniform, and the crystallization of the polyaniline/cobalt oxide catalyst was enhanced, resulting in larger crystal sizes.
Additionally, the polyaniline coating increased the surface area, which implied that there were probably more locations for the electro-conversion of carbon dioxide. The polyaniline/cobalt oxide catalyst had more oxygen vacancies, which trap carbon dioxide and permit the proton-electron transfers required for the transformation, according to an electron pragmatic resonance (EPR) measurement test.
Further experiments were conducted to verify that the enhanced performance of the polyaniline/cobalt oxide catalyst was not solely due to the presence of cobalt oxide or polyaniline. It was the synergistic nature of the polyaniline and cobalt oxide.
In the future, scientists intend to continue enhancing the synergistic performance of cobalt oxide and polyaniline on this catalyst.
“The next step is to optimize the catalyst system, enhancing the tandem effect for better performance. The ultimate goal is the direct electrosynthesis of acetate using carbon dioxide and water as raw materials,” said Wang.
Journal Reference:
Wang, L., et al. (2024) CO2 electroreduction to acetate by enhanced tandem effects of surface intermediate over Co3O4 supported polyaniline catalyst. Carbon Future. doi.org/10.26599/CF.2024.9200013.