Reviewed by Lexie CornerNov 4 2024
Scientists from Kyushu University have demonstrated that singlet fission can be enhanced by introducing chirality—molecules that are non-superimposable on their mirror images—into chromophores and achieving chiral molecular orientation in self-assembled structures. Their findings were published in Advanced Science.
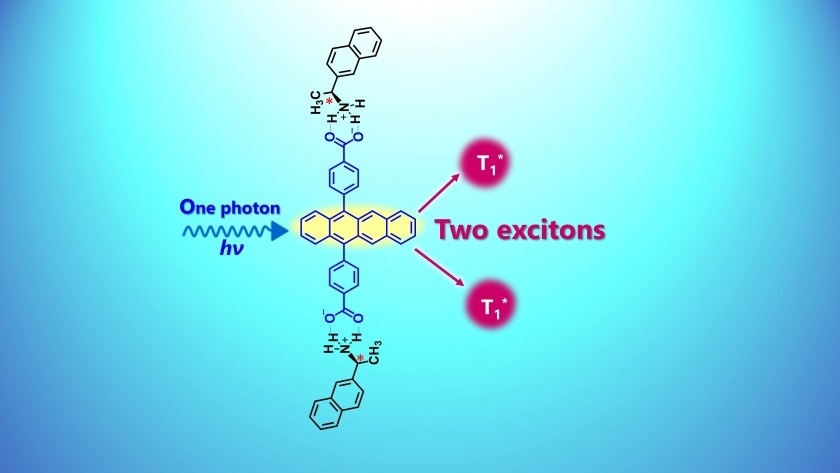 Singlet fission promoted in chiral molecular self-assemblies. Researchers provide a valuable guide for molecular design in singlet fission research. Image Credit: Kyushu University
Singlet fission promoted in chiral molecular self-assemblies. Researchers provide a valuable guide for molecular design in singlet fission research. Image Credit: Kyushu University
An exciton is a particle-bound pair consisting of an electron (with a negative charge) and its corresponding hole (with a positive charge) found in organic compounds. These excitons can move within molecular assemblies and are held together by Coulombic attraction. The process of amplifying an exciton to produce two triplet excitons is known as singlet fission (SF).
This process begins when a single photon, or particle of light, is absorbed by chromophores, specifically designed to absorb certain wavelengths of light. Achieving high SF efficiency in materials with significant potential for optical device applications requires careful control over the molecular orientation and arrangement of these chromophores.
Despite previous experiments on SF conducted in solid materials, comprehensive design guidelines for the molecular organization needed for effective SF are still lacking.
The researchers demonstrated SF-based triplet excitons in self-assembled aqueous nanoparticles containing chiral π-electron chromophores, an observation not seen in comparable racemic nanoparticles (which are mixtures of equal numbers of molecules that are mirror images of each other).
We have discovered a novel method to enhance SF by achieving chiral molecular orientation of chromophores in self-assembled structures.
Nobuo Kimizuka, Professor, Department of Applied Chemistry, Kyushu University
The researchers investigated the SF properties of aqueous nanoparticles formed from ion pairs of tetracene dicarboxylic acid and various chiral or non-chiral amines. They found that the ammonium molecule played a critical role as the counterion—an ion in the solution with a charge opposite to that of another ion.
The counterion significantly influenced several factors, including the strength of intermolecular coupling between tetracene chromophores, structural regularity, spectroscopic characteristics, and the molecular orientation of the ion pairs. As a result, the counterion was essential for regulating the alignment of the chromophores and the associated SF process.
Through extensive experiments using chiral amines, the team achieved a triplet quantum yield of 133 % and a rate constant of 6.99 × 109 s−1. However, they observed that SF did not occur in nanoparticles containing achiral counterions.
Additionally, the racemic ion pair generated an intermediate correlated triplet pair state via SF, but there was no dissociation into free triplets due to the predominance of triplet-triplet annihilation within the triplet pairs.
Our research offers a novel framework for molecular design in SF research and will pave the way for applications in energy science, quantum materials, photocatalysis, and life science involving electron spins. Furthermore, it inspires us to continue exploring SF in chiral molecular assemblies in organic media and thin film systems, which are critical for applications in solar cells and photocatalysts.
Nobuo Kimizuka, Professor, Department of Applied Chemistry, Kyushu University
Journal Reference:
Papadopoulos, I., et al. (2024) Chirality in Singlet Fission: Controlling Singlet Fission in Aqueous Nanoparticles of Tetracenedicarboxylic Acid Ion Pairs. Advanced Science. doi.org/10.1002/advs.202405864.