Tokyo University of Science researchers have created optimized DNA hydrogels with possible biomedical uses by utilizing reduced Takumi-shaped DNA nanostructures. The study was published in the Journal of Controlled Release.
 Takumi-shaped DNA nanostructures form stable DNA hydrogels with high in vivo retention abilities. Researchers optimized Takumi-shaped DNA nanostructures to form efficient hydrogels with minimal DNA units for sustained drug release and high retention abilities. Image Credit: Prof. Makiya Nishikawa from Tokyo University of Science, Japan
Takumi-shaped DNA nanostructures form stable DNA hydrogels with high in vivo retention abilities. Researchers optimized Takumi-shaped DNA nanostructures to form efficient hydrogels with minimal DNA units for sustained drug release and high retention abilities. Image Credit: Prof. Makiya Nishikawa from Tokyo University of Science, Japan
Polymeric materials that contain a lot of water and have three-dimensional network structures are called hydrogels. They function as sustained-release drug delivery systems by encapsulating various bioactive substances, such as drugs, antigens, and cells. Compared to conventional drug delivery methods, hydrogels offer superior biocompatibility, biodegradability, and ease of administration as an injectable scaffold.
Numerous DNA hydrogels have been developed due to DNA's adaptable physicochemical characteristics, drawing much interest as a promising hydrogel material. Current techniques, such as DNA ligase-linked hydrogels, have several drawbacks, such as the possibility of allergic reactions and difficult administration processes that restrict their use in clinical settings.
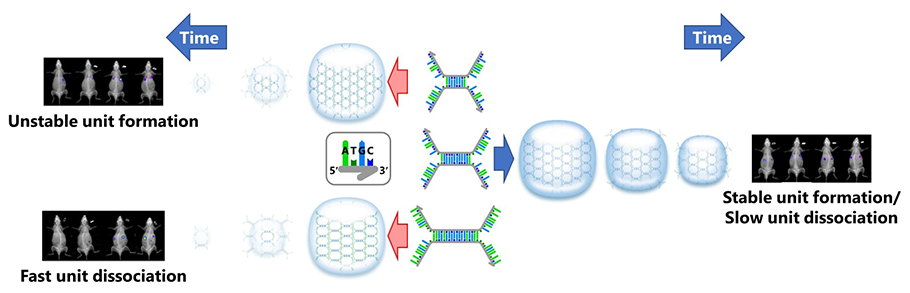
To overcome these difficulties, a polypodna - a nanostructured nucleic acid that resembles a polypod - was recently created by combining three or more premade oligodeoxynucleotides (ODNs) with partially complementary sequences. After being injected, these effective DNA nanostructures easily reform into hydrogels at the injection site.
Although this method produces self-gelatinizing nucleic acids that form hydrogels without needing DNA ligase, it requires multiple ODNs, resulting in high preparation costs, complex design, and a greater likelihood of off-target effects due to the large number of DNA bases involved.
This was addressed using just two ODNs to create a Takumi-shaped DNA unit. Few studies examine its retention capacity or optimization as a sustained-release drug carrier.
These aspects of Takumi-shaped DNA nanostructures were the focus of a recent study by Professor Makiya Nishikawa, Mr. Jian Jin, Assistant Professor Shoko Itakura, and Associate Professor Kosuke Kusamori from Tokyo University of Science, Japan.
Our goal was to miniaturize and optimize DNA nanostructures so that stable DNA hydrogels could be formed with fewer nucleic acids.
Makiya Nishikawa, Professor, Tokyo University of Science, Japan
Each ODN in the Takumi-shaped DNA structure was designed with an 8–18 nucleotide-long palindromic stem, flanked by two cohesive regions on either side, separated by a thymidine (T) spacer. The ODNs form self-dimers through the palindromic sequence, and each ODN is named based on the number of nucleotides in the stem and cohesive regions. For example, 14s-(T-10c)2 denotes an ODN with a 14-nucleotide stem and 10-nucleotide cohesive regions at both ends.
Researchers systematically designed different ODN lengths and examined the relationship between structural characteristics and hydrogel performance, emphasizing in vivo retention capabilities to optimize the Takumi-shaped DNA as an injectable hydrogel unit with sustained retention.
The length of the stem and the cohesive components affect the hydrogel's stability and melting temperatures. ODNs with stem lengths of 12 nucleotides or more effectively created the hydrogel units, indicating that a stem that is 12 nucleotides long is sufficient for unit formation. At 10 nucleotide length, cohesive components also showed good hybridization and interactions.
By varying the length of cohesive parts, the researchers also evaluated the storage modulus of hydrogels, which aids in understanding how the hydrogel changes under various physical conditions. They found that GC-rich cohesive parts that are 10 nucleotides long have superior thermal stability and storage modulus compared to other formations.
The length of the 12s-(T-10c)2-ODN, which showed the highest retention in mice, was 34 bases, requiring only two ODNs to form. In total, just 68 nucleotides were needed for DNA hydrogel formation―markedly lesser than the hexapodna-based DNA hydrogel composed of twelve different 40-base long ODNs.
Makiya Nishikawa, Professor, Tokyo University of Science, Japan
Due to the sustained release of doxorubicin at the injection site, in vivo experiments using doxorubicin-intercalated DNA hydrogels of 12s-(T-10c)2-ODNs demonstrated prolonged persistence of at least 168 hours post-administration, which contributed to pronounced anti-tumor effects in mice. Furthermore, Takumi-shaped DNA hydrogels may trigger specific immune reactions, making them efficient antigen-delivery vehicles.
The optimized DNA hydrogel prepared using 12s-(T-10c)2 exhibited a more sustained retention than the hexapodna-based DNA hydrogel after in vivo administration in mice. These results highlight the applicability of DNA hydrogels as delivery systems for bioactive materials.
Makiya Nishikawa, Professor, Tokyo University of Science, Japan
This study shows that biocompatible hydrogels with long retention periods and sustained drug release capabilities can be created from minimal DNA units, providing a promising biomedical innovation for targeted therapies.
Takumi-Shaped DNS Units Form Hydrogels with Sustained Drug Release
Video Credit: Tokyo University of Science
Journal Reference:
Jin, J., et al. (2025) Biocompatible DNA hydrogel composed of minimized Takumi-shaped DNA nanostructure exhibits sustained retention after in vivo administration. Journal of Controlled Release. doi.org/10.1016/j.jconrel.2024.11.052