Authors
Florian Aubrit*, David Jacob, Sylvain Boj
* actually at Organic Polymers Chemistry Laboratory (LCPO), CNRS-University of Bordeaux, 33600 Pessac, France
Manufacturers require a constant assessment of proteins and active ingredients during injectable pharmaceutical product development (such as vaccines), in order to ensure sufficient product quality. This is especially important when working with proteins that are fragile and prone to modification under conditions like pressure and temperature.
Measurement and Analytics of Protein and API During Process
 Protein aggregation and active principle ingredients (API) stability are factors that ultimately impact both the usability and quality of many biopharmaceutical products, especially injectables.
Protein aggregation and active principle ingredients (API) stability are factors that ultimately impact both the usability and quality of many biopharmaceutical products, especially injectables.
The aggregation of proteins can occur at any stage in a therapeutic protein’s lifecycle, including at the protein expression, refolding, purification, sterilization, shipping, storage, and delivery stages.
A number of manufacturing processes have been found to increase the risk of API degradation and protein aggregation, but in many cases, it is still unknown what the true mechanism is by which these degradations occur.
It is known, however, that chemical degradation of a protein-based product is much more likely when it is exposed to viral or microbial contaminants or stored inappropriately. The effective minimization of these and other key risk factors is vital in helping to prevent or at least mitigate, protein degradation in biopharmaceuticals.
A great deal of research is currently being undertaken with the goal of improving the in situ monitoring of therapeutic protein degradation and denaturation during both production and storage. More rigid health regulations are also being developed, outlining appropriate biopharmaceutical product control measures. The goal of these measures is to ensure protein stability during every stage of the biopharmaceutical lifecycle.
Measurement Techniques Used in Biopharmaceutical Product Characterization
Multi-angle static light scattering (MALS) is a measurement technique that is commonly utilized in the detection of protein aggregates, particularly at an early stage. Some analysts opt to combine MALS with a range of other separating methods; for example, analytical ultracentrifugation, asymmetrical-flow field-flow fractionation, and size-exclusion chromatography (SEC).
Another commonly employed measurement and analytical technique is batch dynamic light scattering (DLS). DLS can be used to provide a characterization of protein aggregates and is often utilized when the shear or dilution encountered in SEC results in disassociation of the protein aggregates.
DLS is also useful when assessing levels of protein aggregation under different environmental conditions, such as temperature. In less than a minute, DLS can facilitate the accurate measurement of particle sizes from one nanometer to up to a few microns.
While these methods are efficient, they often require the sample to be handled and prepared both prior to and during measurement. This handling has the potential to alter the sample aggregation, so researchers must utilize a technique that can accommodate direct analysis of samples in the storage medium, such as in a hermetically sealed syringe or vial.
In Situ Contactless Measurement with VASCO KIN™
 The VASCO KIN™ from Cordouan Technologies offers new possibilities in managing the risk of protein and API modifications. The VASCO KIN™ is a new in situ contactless DLS measurement system, able to accommodate a wide range of containers, including syringes.
The VASCO KIN™ from Cordouan Technologies offers new possibilities in managing the risk of protein and API modifications. The VASCO KIN™ is a new in situ contactless DLS measurement system, able to accommodate a wide range of containers, including syringes.
This innovative instrument is a next-generation time-resolved modality for accurate kinetic analyses, designed to support the characterization of active pharmaceutical ingredients and protein through the real-time monitoring of nanoparticle synthesis, agglomeration, and suspension stability.
The VASCO KIN™ is equipped with an in situ and contactless remote optical head, reducing the risk of contamination considerably. The in-situ head is compact and robust, enabling rapid measurement. It also includes an embedded visible alignment laser, allowing for straightforward installation and precise positioning.
The instrument provides a single and continuous measurement, giving users direct access to a reaction’s full range of characterization data, including scattered intensity, size distribution, correlograms and more.
The VASCO KIN™ is fitted with an ultrafine spectrum-frequency stabilized laser and a high sensitivity artifact-free avalanche photodiode detector. These advanced components combine to deliver high measurement accuracy while accommodating very low scattering samples; for example, samples with very low concentration and/or particles as low as 1 nm in size. A dedicated PC is included, featuring the user-friendly NanoKin® software’s correlation capabilities.
A fully mobile DLS system with a novel Optical Fiber Remote Probe (OFRP) ensures flexibility. The OFRP is highly robust, and is well suited for both direct and contactless measurements with no need for sample batching, thanks to its optimized optomechanical assembly.
The OFRP, is connected to an Optical Unit, and can inject a laser beam into the protein sample, gathering scattered light in a backward direction at an angle of 170 °.
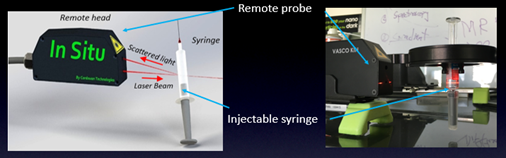
Overall, the VASCO KIN™ is an ideal tool for users who wish to gain deeper insight into protein and API modifications under a range of storage conditions, including syringes and hermetically sealed vials.
In a recent study, a VASCO KIN™ was used for contactless particle size measurements in a series of commercial injectables, including a flu vaccine and a number of protein-based products. An example of one such investigation is displayed here, with a comparison of particle size distribution measurements between an originator and biosimilar injectables from EU and US, which were either expired or not expired.
These results clearly illustrate that measurements supported by a VASCO KIN™ made it possible to accurately compare and discriminate injectable products by their origin, as well as detecting modifications throughout their shelf lifetime.
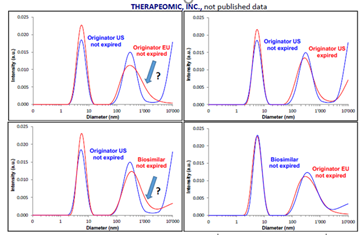
Example of In-situ contactless DLS measurement comparison between originator and biosimilar injectable API products in un-open syringes. Image Credit: Tudor Arvinte & al, Therapeomic Inc, 2018-Not published data.
Conclusion
Proteins and active principal ingredients are susceptible to modification during any stage of the biopharmaceutical product development cycle. Proteins are especially fragile and prone to aggregation, impacting the final product’s overall usability.
The fragility of proteins and other active ingredients necessitates the use of advanced measurement techniques alongside platforms that are able to reliably monitor ingredient stability during the whole product lifecycle.
Due to its suitability for this task, the VASCO KIN™ is currently employed by a range of pharmaceutical companies; utilized for the continuous measurement and monitoring of proteins and other active ingredients across a wide range of biopharmaceutical product development applications.
This platform can facilitate real-time contactless measurements, therefore significantly reducing the risk of disturbing therapeutic samples. It does this while still being able to provide clear visualizations of protein aggregates, the stability of suspensions, nanoparticle synthesis, and modifications in particle sizes.

This information has been sourced, reviewed and adapted from materials provided by Cordouan Technologies.
For more information on this source, please visit Cordouan Technologies.