Sponsored by MerckReviewed by Emily MageeJan 23 2024
Nanometer-sized (2–10 nm) semiconducting crystalline materials known as quantum dots (QDs) display size-dependent optoelectronic properties. For example, the wavelength of their photoluminescence (PL) emission directly correlates with the size of the material as synthesized.
In recent decades, QDs have attracted significant attention as nanoparticle (NP) platforms for diverse applications in electronic devices, bioimaging, biosensing, and drug delivery due to their distinctive features.
Among the notable optical properties of QDs, their large absorption coefficients, substantial stoke shifts, narrow emission bands, high PL quantum yield (QY), and excellent photostability are highly beneficial for biological imaging.1
For instance, a sample can be labeled with QDs of varying sizes and excited with a sole excitation source far removed from the ultraviolet part of the spectrum to generate distinct bright emission colors in the visible, making QDs particularly well-suited for multiplexed imaging.
QDs can be coated with different hydrophilic surface ligands featuring functional chemical groups (such as carboxylic acid and amine) to impart colloidal stability in aqueous environments and facilitate conjugation to biologicals (peptides, ligands, and polymers), dyes, and drugs.
QDs for biological applications usually comprise a core/shell structure capped with hydrophilic ligands. QD systems based on CdSe/ZnS structures are currently the primary research focus due to ease of synthesis and post-synthesis surface modification.
The core of these QDs is usually made of elements from groups II-VI (e.g., CdTe and CdSe), with a shell made of ZnS. The shell protects against core degradation and maintains QD P while additionally acting as an anchor point for ligand attachment.
Despite numerous studies demonstrating stability and the predominantly innocuous nature of Cd-based QD systems in vitro and in vivo,2-4 concerns about the long-term persistence of Cd-containing QDs and associated heavy metal-induced toxicity have driven interest in alternative QD configurations.
Over the past ten years, various research groups have concentrated on synthesizing Cd-free QDs of different sizes, with diverse core/shell configurations and surface coatings. The main aim has been to develop biocompatible alternatives with surface and optoelectronic properties comparable to Cd-based QDs.
Among the non-Cd QDs created, copper indium sulfide (CuInS2), indium phosphide (InP), and graphene QDs have been investigated extensively for biosensing and bioimaging applications.
While many properties of these non-Cd QDs are comparable to Cd-based QDs, their surface chemistry, and colloidal and optical stability in physiological environments have yet to match that of Cd QDs.
This review offers an overview of the current advancements in designing, synthesizing, and surface-modifying strategies for non-Cd QDs for use in bioimaging and sensing applications; with a particular focus on progress made in the last five years, highlighting critical challenges for advancing this field further.
Structural Constructions of Cd-Free QDs
Similar to Cd-based QDs, Cd-free QDs also span sizes of 2–10 nm and consist of core/shell structures. However, the cores of Cd-free QDs commonly feature elements from groups III–V (e.g., InP) (Figure 1).
These cores, particularly InP, possess stronger structural stability and robustness due to their covalent bonds within the matrix.5,6 However, the PL intensity and QY of these cores have generally been lower compared to Cd-based core QDs.
To address this, various studies have utilized shell coatings with broader bandgap material, like ZnS and/or ZnSe layers.
These coatings serve to minimize interactions between the exciton and the core nanocrystal's outer surface to reduce surface defects and quenching; enable better management of emission wavelength, PL lifetime, and QY; and enhance chemical reactivity for ligand exchange.
Depending on the core/shell combination, and the band alignment of the valence and conduction bands of the constituent materials, these QDs can be categorized into three types (type I, type II, and quasi-type II), detailed elsewhere.7
Generally, type I core/shell QDs have a conduction band of the shell at increased energy than that of the core, while the valence band of the shell is at reduced energy compared to the core.
Cd-free type I core/shell QDs, such as InP/ZnS, CuInS2/ZnS, AgInS2/ZnS, and ZnSe/ZnS, have seen widespread development for various biological applications (Figure 1).8
The subsequent section explores some of these examples and emphasizes their role in advancing Cd-free QDs for bioimaging and sensing.
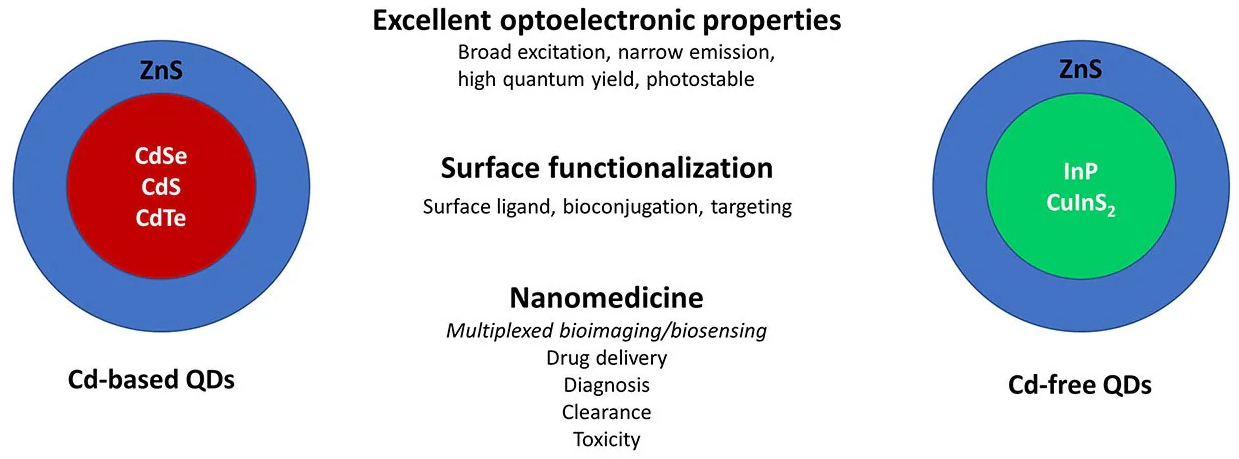
Figure 1. Cd-based vs. Cd-free QDs for applications in nanomedicine. Core/shell QDs based on Cd cores (CdSe, CdS, CdTe) with ZnS have been the prototypical platform for QD-based sensing and imaging. More recently, QDs based on non-Cd cores such as InP and CuInS2 are being investigated as Cd-free options for these applications. Image Credit: Merck
Bioimaging and Sensing With InP and CuInS2 QDs
Among Cd-free QDs, InP/ZnS has emerged as a popular choice for bioimaging.9 Wu et al. linked InP/ZnS QDs to an anti-vascular endothelial growth factor receptor-2 (VEGFR-2) monoclonal antibody and loaded them with miR-92A miRNA to impede the proliferation of cancerous myeloid cells.10
This led to the creation of a bifunctional InP nanocomposite (IMAN) for targeted imaging and therapy. In the assessment of K562 leukemia cell models, using near-infrared (NIR) imaging, the IMAN fluoresced and localized to the plasma membrane before entering the cells.
The researchers illustrated that higher doses of IMAN, incubated with K562 cells for 36 hours, resulted in more efficient cell destruction compared to InP QDs or miR inhibitors alone. In vivo experiments demonstrated the significant impact of VEGFR-2 use on tumor localization specificity.
In K562 tumor-bearing nude mice given tail vein injections of phosphate-buffered saline (PBS), 10 mg/kg InP QDs, or 10 mg/kg IMAN, NIR imaging revealed that while InP QDs diffused within the abdomen, only IMAN localized at the tumor site.
The team also demonstrated that consistent intravenous treatment of 10 mg/kg IMAN over 20 days led to a substantially greater reduction in tumor volume within the mouse model compared to the free miR 92a inhibitor.
Similarly, CuInS2-based QDs have emerged as an additional platform for creating heavy metal-free QDs for bioimaging.
Yang et al. utilized 2-[bis[2-[carboxymethyl-[2-oxo-2-(2 sulfanylethylamino) ethyl]amino]ethyl]amino]acetic acid (DTDTPA)-modified CuInS2/ZnS QDs chelated with gadolinium ions (for magnetic resonance imaging [MRI]) to create a bimodal imaging nanoparticle (QDs@DTDTPA-Gd NPs).
This nanoparticle can be used for NIR fluorescence or MRI.11 DTDTPA was selected due to its water solubility and its ability to chelate gadolinium while attaching to the QD shell.
In a HeLa-tumor-bearing nude mouse model, an increase in magnetic resonance signal intensity within the tumor was observed over 48 hours following an intravenous dose of 20 mg/kg QDs@DTDTPA-Gd NPs. A similar rise in fluorescence intensity from the QD was also noted.
Promisingly, organs harvested from mice after 48 hours revealed that QDs@DTDTPA-Gd NPs concentrated primarily in the liver, tumor, and spleen through passive targeting accumulation. Another research group employed CuInS2/ZnS QDs embedded in a glycol-chitosan matrix (GCM).
After coating with 11-mercaptoundecanoic acid (MUA) to prepare for use in aqueous solutions, the composite was conjugated to n Arg-Gly-Asp (RGD) peptides for enhanced tumor targeting, resulting in cRGDyk-GCM-QDs.12
Examination of in vitro cell viability for GCM-QDs and MUA-QDs using an MTT assay found no significant loss in viability. In vivo, researchers used MRI fluorescence imaging to observe the localization of intravenously injected cRGDyk-GCMQDs at the site of the subcutaneous RR1022 xenograft tumor within nude mice.
Bioimaging and Sensing With Other Types of Cd-Free QDs
Literature reports alternative Cd-free QD configurations, such as an InP core. For instance, Zhang et al. formulated an oil-soluble InP/ZnSe/ZnS core/multi-shell QD. This InP/ZnSe/ZnS QD is unique in its ability to emit at two individual wavelengths with peak emissions in the visible and NIR.13
The surface of the InP/ZnSe/ZnS QDs was modified with a poly(acrylic acid)-octylamine amphiphilic (PAA) polymer grafted with an RGD peptide for water solubility and tumor localization.
In a Bcl-7402 tumor-bearing mouse model, the PAA polymer/RGD-modified QDs were visually located at the tumor site using NIR fluorescence within one hour after intravenous injection of a 1 mg/mL solution of QD.
The control MCF-7 tumor-bearing mouse model, lacking the protein targeted by RGD, did not exhibit targeted localization to the tumor site. The Bcl-7402 mouse model treated with QDs lacking the RGD modification obtained similar results.
While not as widely used as InP/ZnS and CuInS2/ZnS QDs, other types of Cd-free QDs have found applications in physiology. Zn-doped AgInS2 (AIZS) QDs were employed by Zang et al. to make biocompatible AIZS-graphene oxide (AIZS-GO) nanocomposites.14
The process involved assembling oleylamine-modified GO to AIZS QDs via thermal decomposition and mini emulsion. Incubation of AIZS-GO nanoparticles occurred in SK-BR-3 breast cancer cells. A proliferation assay (MTT) evaluated the viability of the cells.
At 0.8 μg/mL, cells maintained 84 % viability, indicating suitable biocompatibility and low cytotoxicity of the AIZS-GO nanocomposites. The team also exhibited the capability to optically image and differentiate AIZS-GO nanoparticles in an SK-BR-3 cancer cell-bearing mouse model.
QDs based on ZnSe core structures serve as another Cd-free option. Zhou et al. utilized manganese-doped ZnSe QDs as a dual MRI/fluorescent imaging probe.15
These Mn-ZnSe QDs were incorporated into mesoporous silica particles to form MSN@QDs, which were then adorned with a tumor-targeting transferrin ligand to create TRF MSN@QDs.
In an in vitro 4T1 cell model, the group observed increased cellular uptake with the transferrin ligand, measured by cellular fluorescence intensity.
Subcutaneous injections of single-QDs, MSN@QDs, and TRF-MSN@QDs (in a nude mouse) and the fluorescence was evaluated.
While single-QDs did not surpass background fluorescence levels, MSN@QDs and TRF-MSN@QDs exhibited significant fluorescence intensity, indicating that enriching the QDs enhanced bioimaging capability.
Critical Considerations, Toxicity, and Future Outlook
Recent advancements in non-Cd QDs for bioimaging and sensing show promise for alternate NP materials in biological applications.
Challenges persist regarding the long-term persistence of core elemental components (such as In, Cu, Se). Similar to Cd-containing QDs, the possible leaching of metal ions from the QD core depends on physicochemical properties like size, shape, and surface ligand functionalization.
These properties dictate the fate of QDs with regard to biodistribution, biodegradation, clearance, and elimination when administered in vivo. The primary technical challenge opposing non-Cd QD development is focused on improved colloidal stability, targeting, and biocompatibility.
Insights from Cd QDs inform the engineering approach, but limited knowledge about the chemistry and synthetic parameters of non-Cd QDs hinders progress.
Challenges remain in creating non-Cd QDs that match the narrow PL bandwidth, high QY, and ease of spectral tuning (UV-NIR) found in Cd QDs. Maintaining these properties upon transferring QDs to water is even more demanding.
Currently, the highest quality Cd QDs for utilization biological applications involve core/shell structures where an inorganic shell (with a wider band gap) encases the core and stops the leaching of potentially toxic metal ions.
ZnS shells are commonly applied to Cd QDs to enhance their PL, make a non-toxic outer surface, and boost both their chemical and photostability.
Optimized biocompatible hydrophilic ligands and bioconjugation strategies have been developed for QDs coated with ZnS. Consequently, QDs without Cd should be adaptable to integrating a ZnS shell, facilitating the adoption of existing technology and simplifying their substitution for Cd-based QDs.
Researchers have concentrated significantly on InP QDs as a less harmful substitute for Cd-based QDs. However, refining their characteristics through chemical synthesis is hindered by their covalent nature, available phosphorus precursors, susceptibility to oxidation, and intricate reaction mechanisms (nucleation and growth).16
Bare InP QDs are infamous for their vulnerability to oxidation while simultaneously displaying subpar QYs. Therefore, it is crucial to overcoat them with passivating inorganic shells like ZnS to improve their PL stability and efficiency.
Despite notable progress in synthesis and shell-coating technology, tuning emission wavelengths while simultaneously yielding bright samples with narrow line widths remains challenging.
Considerable research efforts are being made to perfect the synthesis of InP QDs, comprehend their surface chemistry, and optimize their inorganic surface passivation. CuInS2 QDs encounter similar challenges.
The origin of the emission origin from CuInS2 QDs is yet to be understood, heightening the difficulty of attaining narrow PL from these QDs.17
The development of robust core/shell structures (e.g., CuInS2/ZnS) is made difficult by the rich surface chemistry of CuInS2 QDs, coupled with the influence of precursors and reaction conditions.18
Nevertheless, successfully forming CuInS2 QDs with stable ZnS shells accelerates the testing of surface modifications, augmenting the potential for interfacing these QDs with biological systems.
Conclusion
The adoption of heavy metal-free QDs in biological applications has gained popularity due to their significantly decreased cytotoxicity. Nevertheless, the ongoing challenge persists in creating Cd-free QDs with a high QY and narrow PL.
Though the core/shell model has shown effectiveness in limited in vivo bioimaging using heavy metal-free QDs, these QDs still fall behind their heavy metal-containing counterparts in PL efficiency and QY.
To address this gap, further exploration in QD synthesis and in vitro applications is anticipated. Additionally, there is a foreseen trend in combined methods like core/multi-shell structures and further adjustments after QD synthesis.
Looking ahead, strategies leveraging the QD’s ability to bind ligands on its surface to enhance targeting for in vivo bioimaging are expected. These technologies need to maintain the QD’s imaging capabilities while effectively guiding it through the body to the intended imaging location.
Lastly, even though the use of Cd-free QDs in in vivo bioimaging studies remains limited compared to other approaches in the nanoscience field, an increase in such studies is anticipated in the coming years.
Acknowledgments
The authors express gratitude to the NRL Base Funding Program and the NRL Institute for Nanoscience for their financial backing. K.E.R. is a Ph.D. candidate in the Fischell Department of Bioengineering, University of Maryland College Park.
References and Further Reading
- Zhu C, Chen Z, Gao S, Goh BL, Samsudin IB, Lwe KW, Wu Y, Wu C, Su X. 2019. Recent advances in non-toxic quantum dots and their biomedical applications. Progress in Natural Science: Materials International. 29(6):628-640. https://doi.org/10.1016/j.pnsc.2019.11.007
- Bradburne CE, Delehanty JB, Boeneman Gemmill K, Mei BC, Mattoussi H, Susumu K, Blanco-Canosa JB, Dawson PE, Medintz IL. 2013. Cytotoxicity of Quantum Dots Used forIn VitroCellular Labeling: Role of QD Surface Ligand, Delivery Modality, Cell Type, and Direct Comparison to Organic Fluorophores. Bioconjugate Chem.. 24(9):1570-1583. https://doi.org/10.1021/bc4001917
- Ye L, Yong K, Liu L, Roy I, Hu R, Zhu J, Cai H, Law W, Liu J, Wang K, et al. 2012. A pilot study in non-human primates shows no adverse response to intravenous injection of quantum dots. Nature Nanotech. 7(7):453-458. https://doi.org/10.1038/nnano.2012.74
- Yong K, Law W, Hu R, Ye L, Liu L, Swihart MT, Prasad PN. 2013. Nanotoxicity assessment of quantum dots: from cellular to primate studies. Chem. Soc. Rev.. 42(3):1236-1250. https://doi.org/10.1039/c2cs35392j
- Hussain S, Won N, Nam J, Bang J, Chung H, Kim S. 2009. One-Pot Fabrication of High-Quality InP/ZnS (Core/Shell) Quantum Dots and Their Application to Cellular Imaging. ChemPhysChem. 10(9-10):1466-1470. https://doi.org/10.1002/cphc.200900159
- Bharali DJ, Lucey DW, Jayakumar H, Pudavar HE, Prasad PN. 2005. Folate-Receptor-Mediated Delivery of InP Quantum Dots for Bioimaging Using Confocal and Two-Photon Microscopy. J. Am. Chem. Soc.. 127(32):11364-11371. https://doi.org/10.1021/ja051455x
- Reiss P, Protière M, Li L. 2009. Core/Shell Semiconductor Nanocrystals. Small. 5(2):154-168. https://doi.org/10.1002/smll.200800841
- Nemati A. 2020. Quantum Dots in Therapeutic, Diagnostic and Drug Delivery Applications A Brief Review.. Iran. J. Mater. Sci. Eng. 17(2):1-12.
- Brunetti V, Chibli H, Fiammengo R, Galeone A, Malvindi MA, Vecchio G, Cingolani R, Nadeau JL, Pompa PP. 2009. InP/ZnS as a safer alternative to CdSe/ZnS core/shell quantum dots: in vitro and in vivo toxicity assessment. Nanoscale. 5(1):307-317. https://doi.org/10.1039/c2nr33024e
- Wu Y, Sun J, Zhang Y, Pu M, Zhang G, He N, Zeng X. 2017. Effective Integration of Targeted Tumor Imaging and Therapy Using Functionalized InP QDs with VEGFR2 Monoclonal Antibody and miR-92a Inhibitor. ACS Appl. Mater. Interfaces. 9(15):13068-13078. https://doi.org/10.1021/acsami.7b02641
- Yang Y, Lin L, Jing L, Yue X, Dai Z. 2017. CuInS2/ZnS Quantum Dots Conjugating Gd(III) Chelates for Near-Infrared Fluorescence and Magnetic Resonance Bimodal Imaging. ACS Appl. Mater. Interfaces. 9(28):23450-23457. https://doi.org/10.1021/acsami.7b05867
- Kim E, Lim ST, Sohn M, Jeong H. 2017. Facile synthesis of near-infrared CuInS2/ZnS quantum dots and glycol-chitosan coating for in vivo imaging. J Nanopart Res. 19(7): https://doi.org/10.1007/s11051-017-3944-1
- Zhang J, Wang J, Yan T, Peng Y, Xu D, Deng D. InP/ZnSe/ZnS quantum dots with strong dual emissions: visible excitonic emission and near-infrared surface defect emission and their application in in vitro and in vivo bioimaging. J. Mater. Chem. B. 5(41):8152-8160. https://doi.org/10.1039/c7tb02324c
- Zang Z, Zeng X, Wang M, Hu W, Liu C, Tang X. 2017. Tunable photoluminescence of water-soluble AgInZnS?graphene oxide (GO) nanocomposites and their application in-vivo bioimaging. Sensors and Actuators B: Chemical. 2521179-1186. https://doi.org/10.1016/j.snb.2017.07.144
- Zhou R, Sun S, Li C, Wu L, Hou X, Wu P. 2018. Enriching Mn-Doped ZnSe Quantum Dots onto Mesoporous Silica Nanoparticles for Enhanced Fluorescence/Magnetic Resonance Imaging Dual-Modal Bio-Imaging. ACS Appl. Mater. Interfaces. 10(40):34060-34067. https://doi.org/10.1021/acsami.8b14554
- Tamang S, Lincheneau C, Hermans Y, Jeong S, Reiss P. 2016. Chemistry of InP Nanocrystal Syntheses. Chem. Mater.. 28(8):2491-2506. https://doi.org/10.1021/acs.chemmater.5b05044
- Fuhr AS, Yun HJ, Makarov NS, Li H, McDaniel H, Klimov VI. 2017. Light Emission Mechanisms in CuInS2 Quantum Dots Evaluated by Spectral Electrochemistry. ACS Photonics. 4(10):2425-2435. https://doi.org/10.1021/acsphotonics.7b00560
- Berends AC, van der Stam W, Hofmann JP, Bladt E, Meeldijk JD, Bals S, de Mello Donega C. 2018. Interplay between Surface Chemistry, Precursor Reactivity, and Temperature Determines Outcome of ZnS Shelling Reactions on CuInS2 Nanocrystals. Chem. Mater.. 30(7):2400-2413. https://doi.org/10.1021/acs.chemmater.8b00477

This information has been sourced, reviewed, and adapted from materials provided by Merck.
For more information on this source, please visit Merck.