Sep 9 2019
For the first time, a team of HZB researchers has precisely examined how nanoparticles of lithium sulfide and sulfur precipitate onto battery electrodes when a charging cycle takes place. The results can help improve the service life of lithium-sculpture batteries.
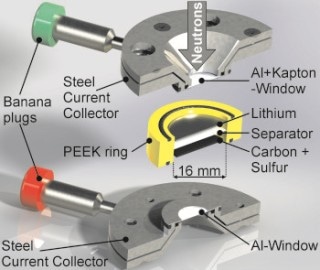 The operando cell was developed at HZB and allows to analyze processes inside the battery during charging cycles with neutrons. (© S. Risse/HZB)
The operando cell was developed at HZB and allows to analyze processes inside the battery during charging cycles with neutrons. (© S. Risse/HZB)
Lithium-sulfur batteries are looked at as one of the most potential candidates for the next generation of energy storage devices. They have a theoretical gravimetric energy density that is five times higher than that of the finest lithium-ion batteries presently available. Plus, they even function at sub-zero temperatures of down to –50 °C. Furthermore, sulfur is economical and eco-friendly.
Capacity Loss
But, so far, their capacity has drastically dropped with every charge-discharge cycle, so that such batteries do not last long anymore. The loss of capacity is induced by complex reaction processes at the electrodes within the battery cell. It is, thus, mainly important to understand precisely how the charge (sulfur) and discharge (lithium sulfide) products precipitate and dissolve.
While sulfur precipitates macroscopically and thus lends itself to inspection by X-ray diffraction or imaging methods during cycling, lithium sulfide is hard to detect because of its sub-10 nm particle size.
“Operando” Observations with Neutrons
Now, for the first time, a better understanding of this has been provided by explorations with the BER II neutron source at the HZB. Dr Sebastian Risse used a measuring cell which he created to illuminate lithium-sulfur batteries with neutrons during charging and discharging cycles (operando) and concurrently carried out extra measurements using impedance spectroscopy.
This allowed him and his team to inspect the dissolution and precipitation of lithium sulfide with great precision during 10 discharge/charging cycles. Since neutrons interact intensely with deuterium (heavy hydrogen), the scientists used a deuterated electrolyte in the battery cell to render both the solid products (sulfur and lithium sulfide) visible.
Surprising Insight
We observed that the lithium sulphide and sulphur precipitation does not take place inside the microporous carbon electrodes, but instead on the outer surface of the carbon fibres.
Dr rer. nat. Sebastian Risse, HZB
These results offer a valuable guide for developing better battery electrodes.
The research has been published in ACS Nano.