When developing electrocatalysts effectively, a thorough understanding of the physical properties at the solid-liquid interface is necessary. Advancements in atomic force microscopy (AFM) could enable the imaging of the height profile of nanostructures, as well as the electric current and frictional force at this interface.
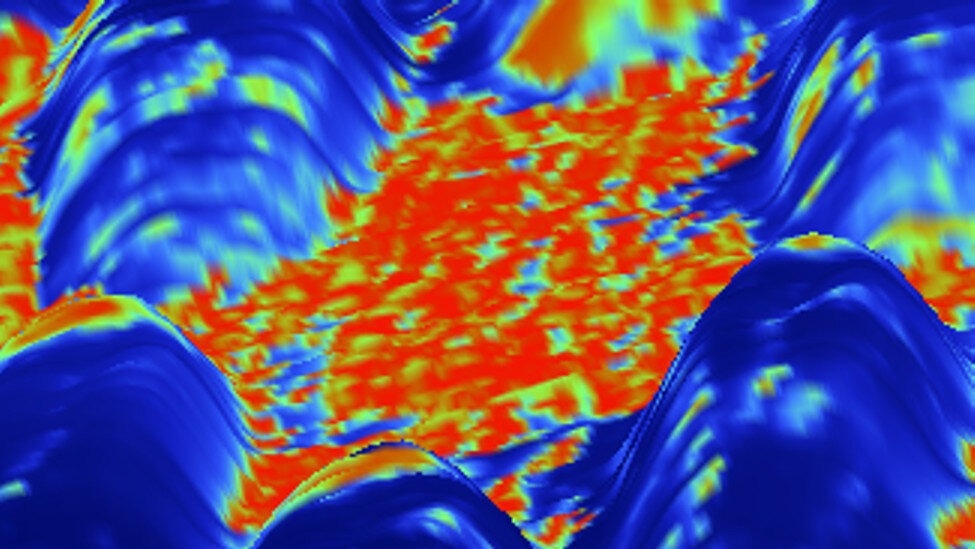
An overlay of the current signal on a three-dimensional representation of the height image. This clearly shows island-like regions.The newly developed method was used to scan the surface of a bimetallic catalyst material in an aqueous medium. The figure shows an overlay of the current signal on a three-dimensional representation of the height image. This clearly shows island-like regions. Credit: M.Munz/FHI/HZB
A recent study published in the Journal of the American Chemical Society has successfully evaluated electrocatalytically active materials and obtained valuable insights that can facilitate the optimization of electrocatalysts. Additionally, this method shows potential in exploring the mechanisms of battery electrodes, photocatalysis, and active biomaterials.
Determining Properties of Solid-Liquid Interfaces
The local features of solid-liquid interfaces impact the overall efficacy of important catalytic processes, such as the electro-catalytic conversion of carbon dioxide, hydrogen, and oxygen, in addition to battery-based electrolytic energy storage.
Developing reliable structure-property connections under liquid-phase reaction conditions is critical for the design of sophisticated (photo)electrocatalysts. However, there are several challenges associated with this, such as precisely mapping electrical impulses in photoelectrochemical systems and identifying corrosion spots and substance aging processes.
Moreover, understanding the interfacial arrangement of water molecules and electrolytic ions, as well as their impact on interfacial electron transport, is critical for improving electrochemical microenvironments.
Correlative in situ analysis is crucial for examining the interactions between mechanical, electric, and electrochemical characteristics at length scales comparable with the distinctive interfacial features to solve these problems.
Atomic Force Microscopy for Analysis of Solid-Liquid Interfaces
Conductive atomic force microscopy (c-AFM) is a powerful technique for imaging local variations in electric conductivity on surfaces, offering high lateral resolution in contact mode. This makes it a promising tool for in situ correlative imaging of solid-liquid interfaces.
The accuracy of present imaging in contact modes is affected by several variables, including load, tip radius, local adhesive force, and surface distortion. Although most previous studies were done in air or vacuum, other studies used nonpolar liquids to generate inert conditions.
However, there is still much to explore with c-AFM, particularly in polar liquids related to electrocatalysis. To date, there have been no reports on using c-AFM imaging in polar liquids in conjunction with friction force imaging.
Nonetheless, the technique holds great potential for studying a wide range of phenomena, from frictional properties at solid-liquid interfaces to optimizing catalysts.
Highlights of the Current Study
In this study, the researchers utilized conductive atomic force microscopy (c-AFM) to investigate the potential of nanostructured and bimetallic copper-gold electrocatalysts for carbon-dioxide electroreduction.
The research aimed to give nanoscale insights into the relationship between local current and friction force, which is influenced by molecular hydration layer stacking at the catalyst-electrolyte-probe interface.
In the experiment, an extremely sharp tip was used to scan across the surface of the catalyst, and the tip's height profile was recorded. The tip was attached to a miniaturized cantilever, enabling the measurement of force interactions between the tip and the sample surface, including frictional forces, with high sensitivity.
"This allowed us to simultaneously determine the electrical conductivity, the mechanical-chemical friction and the morphological properties in situ (i.e., under the relevant liquid-phase conditions rather than in vacuum or in air)," emphasizes Christopher Kley, a co-author of the study.
Future Prospects
The researchers introduced an innovative in situ correlative atomic force microscopy approach that enables simultaneous imaging of an electrocatalyst's local electrical, chemical-frictional, and morphological properties in aqueous media and under potential control.
By studying bimetallic gold-copper electrocatalysts, researchers could observe islands of copper oxide with higher electrical resistance, grain boundaries, and low-conductivity regions in the hydration layer.
Such results on solid-liquid interfaces help to optimize them in a targeted manner. The researchers also identified the local electrochemical environments that influence charge transfer at the interface.
The study shows that in situ conductive AFM combined with lateral force microscopy is broadly applicable to the nanoscale characterization of electrified solid-liquid interfaces.
This research is valuable for energy research, battery systems and in other fields like corrosion processes, nanosensor systems, and fluidics and environmental sciences. Further research in this field has the potential to improve understanding of electrochemical conversion processes, which can play a critical role in the development of energy-efficient technologies.
Reference
Munz, M. et al. (2023). Nanoscale Electron Transfer Variations at Electrocatalyst–Electrolyte Interfaces Resolved by in Situ Conductive Atomic Force Microscopy. Journal of the American Chemical Society. Available at: https://doi.org/10.1021/jacs.2c12617
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.