Advances in nanotechnology have significantly influenced drug delivery systems, enabling new approaches to improve bioavailability, stability, and targeted delivery. Lipid nanoparticles (LNPs) and liposomes have become important tools in addressing key challenges in this field.
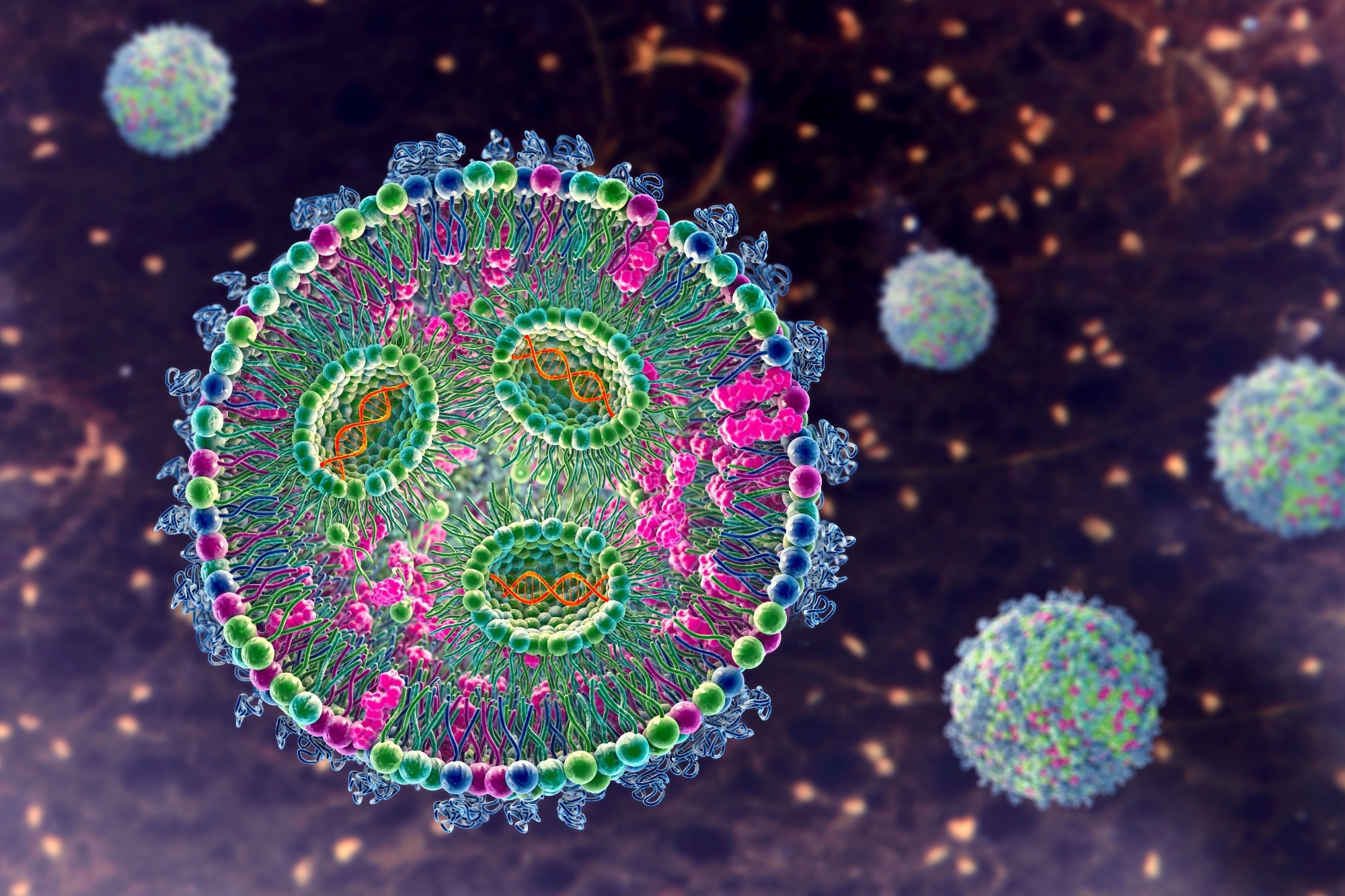
Image Credit: Kateryna Kon/Shutterstock.com
While both are lipid-based and share some functional similarities, their structural and functional differences have positioned them for specific applications in the pharmaceutical industry. This article provides a detailed overview of these differences, exploring their design, functionality, applications, and the advantages and limitations associated with their use in healthcare.
What is the Difference Between Liposomes and Lipid Nanoparticles?
Structural and Compositional Differences
LNPs are solid or semi-solid particles at room temperature. They are composed of a lipid core stabilized by surfactants or other agents. This lipid core provides a hydrophobic environment ideal for encapsulating genetic materials such as messenger RNA (mRNA) or small interfering RNA (siRNA).
LNPs typically contain ionizable lipids, cholesterol, phospholipids, and polyethylene glycol (PEG), which collectively support efficient endosomal escape, ensuring the therapeutic payload reaches its intracellular target.1,2
Liposomes are spherical vesicles formed by one or more lipid bilayers surrounding an aqueous core. This structure allows them to encapsulate both hydrophilic drugs (in the aqueous core) and hydrophobic drugs (within the lipid bilayer), making them versatile for various therapeutic agents.
Liposomes primarily comprise phospholipids and cholesterol, contributing to their stability and controlled drug release. However, compared to LNPs, they are less efficient in delivering drugs directly into cells, favoring extracellular or gradual drug release.1,3
Size and Stability
Size and stability are critical factors in the efficacy of drug delivery systems. LNPs LNPs are typically smaller, ranging from 20 to 100 nanometers. Their compact design provides high stability during storage, reducing aggregation and degradation of encapsulated drugs. This stability is particularly crucial for genetic therapies, where maintaining the integrity of the payload is essential.1,2
Liposomes are larger, with sizes ranging from 50 to 1000 nanometers. While their larger size allows them to carry more therapeutic agents, it also increases the risk of leakage and degradation, especially during storage. To mitigate these issues, liposomes are often coated with PEG or other stabilizing agents to enhance their durability and extend their circulation time within the body.1,3
Drug Delivery Applications
The distinct properties of LNPs and liposomes lend themselves to diverse drug delivery applications.
LNPs
LNPs have been pivotal in the development of mRNA-based vaccines, such as the Pfizer-BioNTech and Moderna COVID-19 vaccines. These vaccines use LNPs to encapsulate and protect fragile mRNA, ensuring its safe delivery to target cells, where it elicits an immune response.2,4
However, they can trigger unwanted immune responses, leading to potential side effects. Manufacturing consistency and optimizing how they release drugs within cells are other key challenges that need attention.2,4
Beyond vaccines, LNPs are being extensively studied for gene-silencing therapies using small interfering RNA (siRNA) and for delivering CRISPR-Cas9 components in gene-editing applications. Researchers are also investigating their use in cancer immunotherapy, where LNPs can deliver tumor antigens or immunostimulatory agents to enhance the body’s immune response against cancer cells.2,4
Liposomes
Liposomes are widely used to encapsulate various therapeutic agents. Their ability to carry both water- and fat-soluble drugs makes them incredibly versatile, especially for combination therapies. They have a long history of use in medicine, with well-established formulations for cancer and fungal infections.
However, they are prone to leakage and degradation during storage, and their circulation time in the body is limited unless modified. Large-scale production of liposomes can also be challenging, as maintaining uniform size and drug loading is technically demanding.3,5
In oncology, liposomal formulations of drugs like doxorubicin (such as Doxil®) enhance drug accumulation in tumor tissues through the enhanced permeability and retention (EPR) effect while minimizing cardiotoxicity.3,5
Liposomes are also used in antifungal therapies, such as Ambisome®, to deliver amphotericin B with reduced toxicity. In vaccine development, liposomes can act as carriers for antigens and adjuvants, improving immune responses. They are also being explored for delivering monoclonal antibodies, offering targeted therapy options for autoimmune diseases and certain types of cancers.3,5
Addressing Key Challenges in LNP and Liposome-Based Drug Delivery
Researchers are developing practical approaches to address the challenges of using LNPs and liposomes in drug delivery.
For LNPs, microfluidic technologies are being used to control particle size and ensure consistent quality during manufacturing. Efforts to design new ionizable lipids aim to reduce immune responses and improve the efficiency of drug release within cells. Automating production processes is another focus to meet the increasing demand for LNP-based medicines, particularly vaccines.4
Biodegradable LNPs are also under investigation to reduce toxicity and improve compatibility with the body. A recent study in Nature Communications introduced biodegradable LNPs with alkyne and ester modifications, enhancing mRNA delivery, reducing toxicity, and improving cellular uptake and membrane fusion. These advancements aim to provide safer and more effective non-viral mRNA delivery systems.6
For liposomes, research focuses on improving stability and extending circulation time in the body. Adding stabilizing agents to the lipid bilayer or modifying the surface with ligands can help target specific tissues and minimize drug leakage. Manufacturing processes, such as high-pressure homogenization, are also being refined to scale up production while maintaining quality.5
Integrating liposome technology with personalized medicine offers further potential for tailoring treatments to individual patients. A study in ACS Applied Materials & Interfaces introduced specialized liposomes for MRI-guided cancer therapy. These liposomes, incorporating targeted and traceable lipids, enabled multi-stimulus-controlled drug release and significantly enhanced tumor suppression. Such approaches demonstrate the potential for precision drug delivery with improved outcomes and minimized side effects.7
The Future of LNPs and Liposomes in Drug Delivery System
LNPs and liposomes represent distinct but complementary approaches to drug delivery. LNPs are particularly effective in delivering genetic materials like mRNA and siRNA, making them integral to the development of gene therapies and vaccines. Liposomes, on the other hand, are versatile carriers suitable for a wide range of therapeutic agents, including chemotherapeutics and antifungals.
Understanding their unique features, benefits, and limitations is essential to maximize their potential in medical applications. As research continues, these lipid-based systems are expected to be increasingly significant in advancing drug delivery and improving therapeutic outcomes.
More from AZoNano: The Evolution of Lipid Nanoparticles
References and Further Reading
- Viegas, C. et al. (2023). Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics. DOI:10.3390/pharmaceutics15061593. https://www.mdpi.com/1999-4923/15/6/1593
- Bukke, SPN. et al. (2024). Solid lipid nanocarriers for drug delivery: design innovations and characterization strategies—a comprehensive review. Discov Appl Sci. DOI:10.1007/s42452-024-05897-z. https://link.springer.com/article/10.1007/s42452-024-05897-z
- Liu, P. et al. (2021). A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules. DOI:10.3390/molecules27041372. https://www.mdpi.com/1420-3049/27/4/1372
- Dhayalan, M. et al. (2024). Advances in functional lipid nanoparticles: from drug delivery platforms to clinical applications. 3 Biotech. DOI:10.1007/s13205-023-03901-8. https://link.springer.com/article/10.1007/s13205-023-03901-8
- Jiang, Y. et al. (2023). Lipid-Based Nanotechnology: Liposome. Pharmaceutics. DOI:10.3390/pharmaceutics16010034. https://www.mdpi.com/1999-4923/16/1/34
- Miao, L. et al. (2020). Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nature Communications. DOI:10.1038/s41467-020-16248-y. https://www.nature.com/articles/s41467-020-16248-y
- Liu, C. et al. (2021). Magnetic Resonance Imaging-Guided Multi-Stimulus-Responsive Drug Delivery Strategy for Personalized and Precise Cancer Treatment. ACS Applied Materials & Interfaces. DOI:10.1021/acsami.1c13853. https://pubs.acs.org/doi/full/10.1021/acsami.1c13853
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.