Lipid nanoparticles (LNPs) are emerging as potential carriers for delivering an array of therapeutic compounds in the field of medicine. This article will provide an overview of lipid nanoparticle formulation, emphasizing its importance in the nanoscience field.
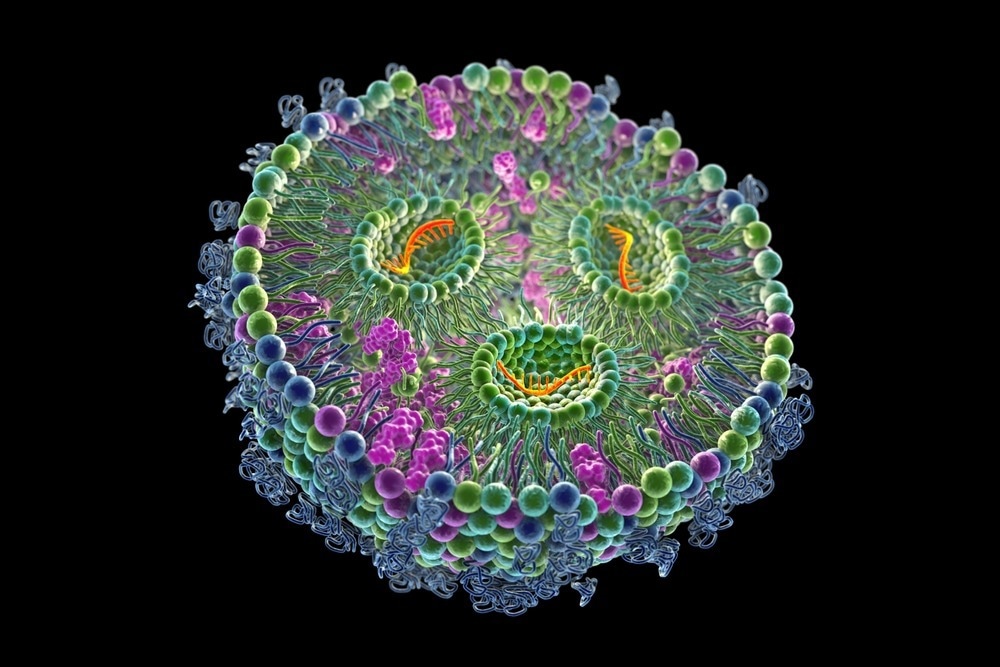
Image Credit: Kateryna Kon/Shutterstock.com
Brief Overview of LNPs
Lipid nanoparticle formulation has increased over the past two decades. LNPs have proven to be effective nano-based delivery vehicles for cytotoxic chemotherapeutic drugs, nucleic acid therapies and antibiotics.
Lipid nanoparticle formulation has several advantages, including protecting therapeutic drugs from in-vivo breakdown, increasing their efficacy and solubility, improving intracellular permeability, increasing drug bioavailability, controlling the active compound release, and controlling drug delivery for specific sites.
Since the initial FDA clearance of Doxil; doxorubicin (DOX) loaded PEGylated liposome for potential treatment of cancers of the breast, ovary, and solid tumors in 1995, numerous lipid nanoparticle formulation-based drug delivery systems (DDS) have been designed and accomplished clinical success.
Other disorders, such as tuberculosis, asthma, and infection, have also been targeted with drug-loaded lipid nanoparticle formulation. To treat tuberculosis, for example, a muco-adhesive rifampicin-loaded lipid nanoparticle formulation approach was created, which displayed increased mucoadhesive strength and greater cellular absorption.
In gene therapy, lipid nanoparticle formulation can be employed as synthetic or nonviral carriers to treat hereditary and acquired illnesses. A lipid nanoparticle formulation has recently been created as a critical component of COVID-19 mRNA vaccines, where LNPs serve a major role in efficiently maintaining and transferring mRNA to cells.
Lipid nanoparticle formulation has also been used in various different fields such as cosmetics, medical imaging, agriculture, nutrition, and other cutting-edge technologies such as nanoreactors.
Basic Principles of Lipid Nanoparticle Formulation
LNPs are colloidal particles made up of a surfactant-stabilized lipid matrix. Liposomes, solid-lipid nanoparticles, lipid nanoemulsions, nanostructured lipid carriers, and lipid polymer hybrid nanoparticles are the five subcategories of LNPs.
A lipid nanoparticle formulation is stable at ambient temperature and ranges in size from 40 nm to 1000 nm. Small-sized LNPs (size <100 nm) have faster cellular absorption and longer circulation periods, whereas large-sized LNPs (size >100 nm) might possess an increased drug-loading capacity.
Solid lipids, surfactants, co-surfactants (when required), and active compounds make up lipid nanoparticle formulation-based nanocarriers. The lipid content in a lipid nanoparticle formulation is important.
Cholesterol and phospholipids are essential for lipid nanoparticle formulation and stability; ionizable or cationic lipids allow binding with negative-charged nucleic acids, improving drug loading; and PEGylated lipids help regulate circulation time and particle stability inside a biological system.
LNP size, surface charge, stability, shape, loading efficiency, and entrapment efficacy are all important aspects of any lipid nanoparticle formulation.
The surface charge of a lipid nanoparticle formulation is a significant metric that determines suspension stability and is often measured via zeta potential studies.
A significant surface charge is indicated by a highly negative or positive zeta potential, resulting in electrostatic repulsion among particles and an increase in their dispersion stability.
The mechanism of action of a lipid nanoparticle formulation during cellular delivery is due to its biomimetic design and the resemblance of its lipid molecules to that of cellular membranes. LNPs generally pass the cell membrane to distribute nanoparticle contents to intracellular locations.
LNPs make use of the weak pH environment inside the target cells upon passing the cell membrane, encouraging endosomal escape along with the release of the therapeutic loads into the cytoplasm.
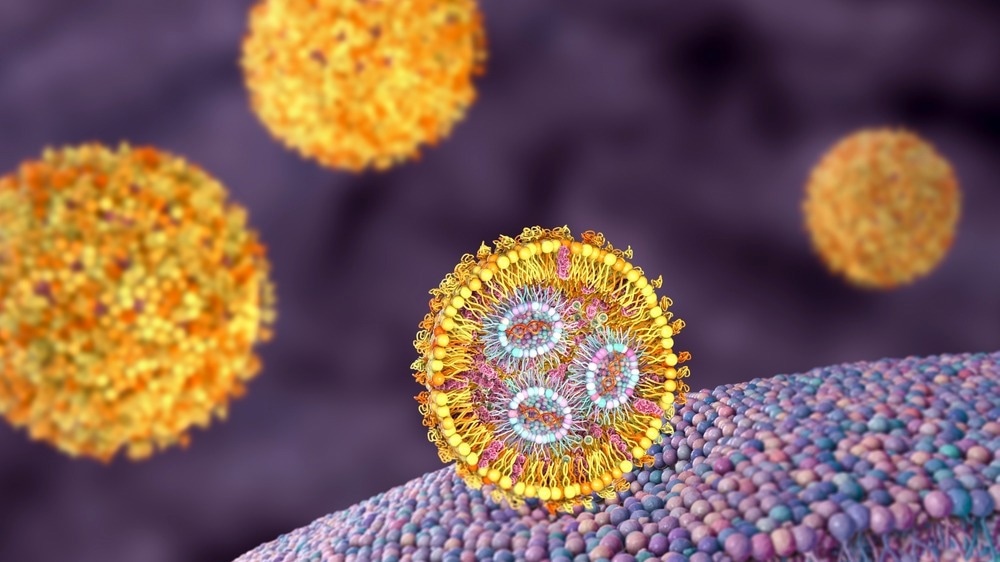
Image Credit: Kateryna Kon/Shutterstock.com
Methods of Lipid Nanoparticle Formulation
The synthesis of lipid nanoparticle formulation represented a significant step forward in pursuing the next generation of nanocarriers. The methods of lipid nanoparticle formulation described below are frequently employed.
Nanoprecipitation / Solvent displacement
A water-miscible solvent comprising hydrophobic drugs and lipids, known as the organic phase, is mixed with the water phase in this method of lipid nanoparticle formulation. The organic and aqueous phases combine under magnetic conditions.
Rapid lipid and drug dissolution results in fast precipitation of lipid nanoparticles and prompt drug encapsulation.
In a lipid nanoparticle formulation, variables including organic solvent/antisolvent ratio, stirring rate and lipid/surfactant/drug concentrations can be changed to control the nanoparticle size and drug encapsulation efficacy.
Microfluidic Approaches
This method of lipid nanoparticle formulation involves the precise mixing of therapeutic drug and lipid solutions in the microchannels of a microfluidic device.
For lipid nanoparticle formulation, two types of microfluidic devices are used: chip-based platforms, which entail the precisely controlled blending of organic solvents and aqueous solvents inside a customized chip, or capillary-based platforms, which entail the introduction of the organic solvent via a central tube encircled by several tubes for fast dilution of the escaping aqueous solution.
The microfluidic technique enables fine control of lipid nanoparticle formulation method variables like flow rates and mixing ratios to enhance LNP properties.
Compared to bulk mixing techniques, microfluidics allows for more consistent and repeatable lipid nanoparticle formulation.
Thin Film Hydration
In this lipid nanoparticle formulation method, lipids are dispersed and blended in an organic solvent to attain homogeneity, after which the solvent is evaporated via rotary evaporation, generating a thin and fine lipid film that covers the flask's walls.
The lipid suspension is hydrated before being passed via a filter having uniform pore diameters numerous times, leading to homogeneous-sized lipid nanoparticles.
In comparison to the thin film hydration method of lipid nanoparticle formulation, microfluidics offer better encapsulation efficiency and scalability. In terms of homogeneity, thin film hydration and nanoprecipitation offer Polydispersity index (PDI) ~ 0.5 and microfluidics method of lipid nanoparticle formulation offers great PDI <0.2.
The research resulted in the development of a commercially viable lipid nanoparticle formulation. Examples of commercial lipid nanoparticle formulation companies include LipExoGen Biotech.
They provide lipid nanoparticle formulation solutions for preclinical studies. Liposomes, solid lipid nanoparticles, micelles, and polymeric nanoparticles are among their specialties, along with various other lipid nanoparticle formulation-based drug delivery platforms.
Challenges and Limitations in Lipid Nanoparticle Formulation
Though the lipid nanoparticle formulation is low in toxicity, has good conformance with biological substances, and most preparation processes are easily scaled up, there are some scale-up issues and constraints that must be examined and addressed.
Stability is a vital requirement in the industrial manufacturing of any lipid nanoparticle formulation and it can be impacted through lipid polymorphism, which relates to phase changes.
Due to being susceptible to chemical and physical changes during storage, industrial production of lipid nanoparticle formulation poses considerable hurdles.
These modifications can have a negative impact on drug encapsulation, release qualities, and stability, resulting in aggregation, size distribution alterations, and drug leakage.
Several approaches have been developed to address these issues, including lyophilization, the introduction of stabilizing chemicals such as antioxidants to avoid oxidation, the use of additives as buffers, and specific packing materials to inhibit container interactions.
In addition to the obstacles of research development, the regulatory approval procedure is expected to be a hurdle for unsuitable lipid nanoparticle formulation-based solutions.
LNPs must meet safety, quality, and efficacy standards before being approved by regulatory authorities like the Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
For complete regulatory compliance, toxicology tests are critical, especially when it comes to lipid nanoparticle formulation because the buildup in normal tissues might result in genotoxicity and cytotoxicity.
Future Outlook
Lipid nanoparticle formulation has shown potential as therapeutic nanocarriers, generating considerable demand in a variety of fields, particularly in the field of nanoscience.
LNPs offer improvement in drug delivery. Various forms and methods of lipid nanoparticle formulation provide unique benefits and issues that need careful consideration when developing drug delivery systems.
While clinical trials provide proof that lipid nanoparticle formulation is effective, more advancements in research and development are urged to assure safe and efficacious drug delivery.
References and Further Reading
Anon. Liposomal Formulation Development — LipExoGen. Available at: https://lipexogen.com/services/custom-liposome-synthesis/liposomal-formulation-development/ (Accessed Dec. 11, 2023).
Mehta, M., et al. (2023). Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development. ACS Materials Au. doi.org/10.1021/acsmaterialsau.3c00032.
Xu, L., et al. (2022). Lipid Nanoparticles for Drug Delivery. Advanced NanoBiomed Research, 2(2), p.2100109, doi.org/10.1002/ANBR.202100109.
Inside Therapeutics. Lipid nanoparticles: a complete guide to understanding LNP. Available at: https://insidetx.com/review/complete-guide-to-understanding-lipid-nanoparticles/ (accessed Dec. 11, 2023).
Zhao, Y., & Huang, L. (2014). Lipid Nanoparticles for Gene Delivery. Advanced Genetics, vol. 88, p. 13, doi.org/10.1016/B978-0-12-800148-6.00002-X.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.