A study recently published in the journal Catalysts describes a procedure to inexpensively manufacture the carbon allotrope, fullerenes, by electrolysis of carbon dioxide (CO2) with carbonate in molten form.

Study: Controlled Growth of Unusual Nanocarbon Allotropes by Molten Electrolysis of CO2. Image Credit: acinquantadue/Shutterstock.com
Carbon Dioxide – The Greenhouse Gas
The greenhouse gas, carbon dioxide, absorbs and reflects heat.
The Earth constantly emits heat signatures in the form of infrared radiation, which are be trapped in the upper atmosphere by the carbon dioxide and reflected back to the Earth’s surface and oceans. This causes the temperature of the Earth to rise.
Unlike its counterparts, nitrogen or oxygen, which amount to more than 99% of our atmosphere, greenhouse gases absorb the heat and then slowly emit them much like the warmth of coal after they have been burned out.
The Earth's average yearly temperature would be lower than zero without this atmospheric carbon dioxide.
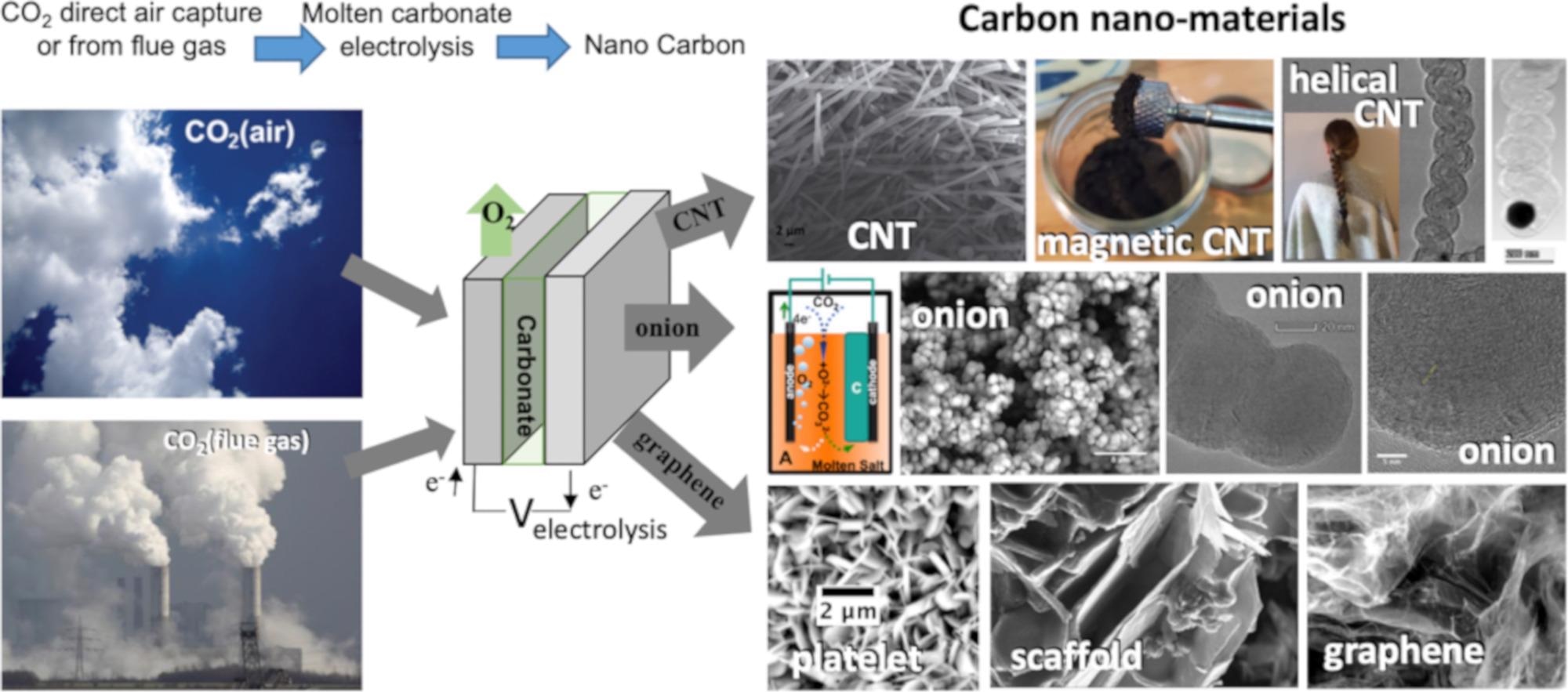
Figure 1. High-yield electrolytic synthesis of carbon nanomaterials from CO2, either directly from the air or from smokestack CO2, in molten carbonate. © Liu, X., Licht, G., Wang, X., & Licht, S. (2022)
Increases in greenhouse gases, on the other hand, have thrown the Earth's energy budget off-kilter, trapping more heat and raising the planet's average temperature.
If things continue to progress at this rate, carbon dioxide will lead the planet to warmer temperatures, more abrupt climatic changes and global catastrophes. Thus, while global policies are trying to encourage lesser carbon footprints, scientists are hard at making use of the CO2 already present in the air to try to utilize it for practical purposes.
CO2 was formerly assumed to be non-reactive. In this study, researchers found a use for carbon dioxide as a synthesis with molten carbonate to use them for graphitic carbon nanomaterials (CNM).
Graphitic Carbon Nanomaterials – An Introduction
A huge number of unusual nanocarbon formations exist outside of the realm of standard graphite, diamond, and buckyballs.
Graphitic carbon nanomaterials (CNM) are high-value, extremely stable (geologic stability similar to graphite) state-of-the-art materials with the potential to be appealing CO2 conversion products.
While CNMs by CO2 are already under consideration by the scientific community, current methods, such as carbon vapour deposition, to produce these materials leave a much higher carbon footprint than it uses effectively for conversion. Furthermore, commercial production of nanocarbons is highly expensive and leaves a high carbon footprint, thus affecting the environment adversely.
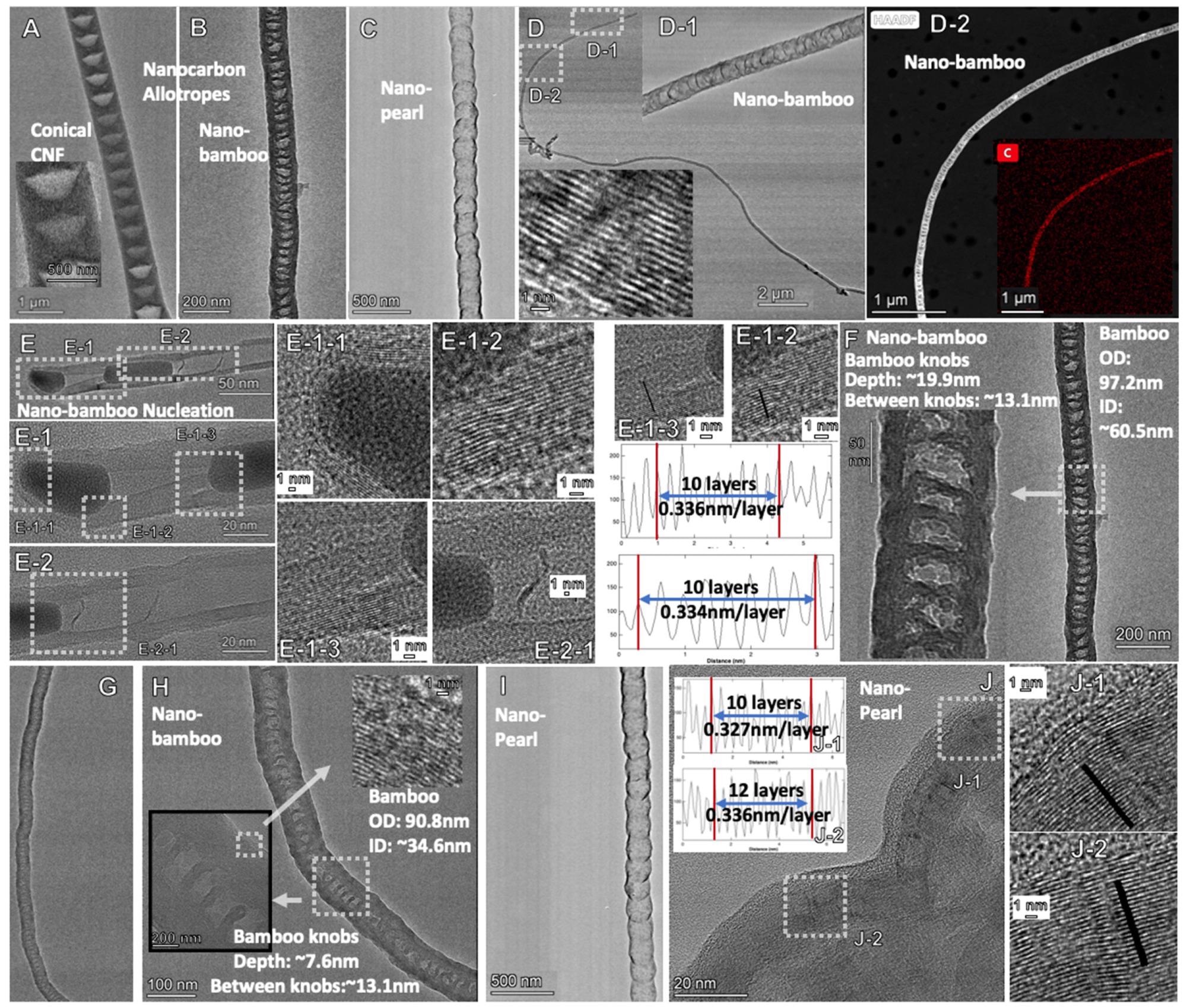
Figure 2. TEM of new molten carbonate synthesized carbon allotropes: Comparison of nano-bamboo, nano-pearl and conical CNF. (A): CNF; (B): Nano-bambpo; (C): Nano-pearl; (D), D-1, D-2 Nanobamboo; (E), E-1, E-2, E-1-1; E1-2 & E1-3 Nano-bamboo including measured graphene layer thickness; (F–H) Nano-bamboo knobs, (I,J) Nano-pearl; J-1, J-2 Nano-pearl with measured graphene layer thickness. © Liu, X., Licht, G., Wang, X., & Licht, S. (2022)
Converting CO2 into Graphitic Carbon Nanomaterial
CO2 may be changed into a variety of carbon morphologies.
The ability to make these allotropic forms at high purity using a simple electrolysis method is similar to how aluminum is made by splitting aluminum oxide, but instead of aluminum, nanocarbon is made by splitting CO2, which opens up a world of new materials with exciting new properties such as high physical strength, flexibility, robustness, conductivity and electrical and thermal properties.
The team referred to these nanomaterials as fullerene allotropes, which are manufactured by the reaction of carbonate in molten form with the carbon dioxide we see in the atmosphere.
The team showed that through the use of CO2 as a reactant in the production of value-added CNM items, people could be encouraged to use this greenhouse gas to combat climate change, and its transformation into CNMs can offer a stable material for storing carbon that has been removed from the environment.
Morphologies of Obtained Samples
The research group obtained different morphologies from the carbon dioxide samples and observed interesting properties for the alloys with different morphologies.
These configurations were given different names relating to the orientation of the carbon atoms in their structure, with examples including nano-bamboo, nano-belt and nano-trees.
Such distinct morphologies may give rise to extraordinary physical and chemical characteristics with potential implications beneficial to applications like those that rely on high tensile strength and high heat capacity. These properties are disseminated differently all through the allotropes because of their geometries.
The Present and the Future
The creation of a collection of rare, valuable nanocarbon allotropes is made possible by molten carbonate electrolysis of CO2. The mass construction of these nanostructures from CO2 will give an important incentive for people to utilize this greenhouse gas.
Such nanostructures are uncommon, if not non-existent, and thus are not widely available commercially.
Should these procedures be widely employed after rigorous scientific analysis, it would be possible to make use of carbon dioxide to turn it into a scientifically and commercially viable product. However, many efforts are needed to mitigate the greenhouse effect as these procedures will only be able to remove a fraction of CO2 from the atmosphere.
Continue reading: How are Carbon Nanotubes Made from Graphene?
Reference
Liu, X., Licht, G., Wang, X., & Licht, S. (2022). Controlled Growth of Unusual Nanocarbon Allotropes by Molten Electrolysis of CO2. Catalysts, 12(2). Available at: https://www.mdpi.com/2073-4344/12/2/125
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.