A research team from Rice University has resolved a long-standing debate on how silver nanoparticles kill bacteria.
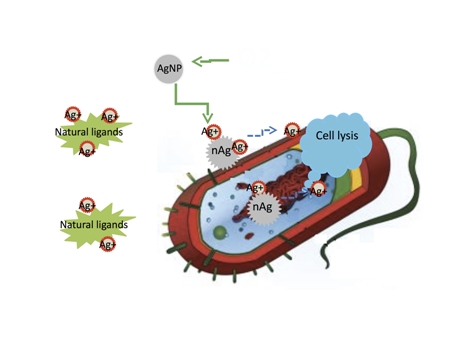 New research by Rice University found that silver ions, not the particles themselves, are toxic to bacteria. They also found that ligands in the vicinity of a bacteria can bind silver ions, preventing them from reaching their target. (graphic by Zongming Xiu)
New research by Rice University found that silver ions, not the particles themselves, are toxic to bacteria. They also found that ligands in the vicinity of a bacteria can bind silver ions, preventing them from reaching their target. (graphic by Zongming Xiu)
Researchers have long known the antimicrobial properties of silver ions, which are released during oxidation of nanoparticles. However, they have also assumed that silver nanoparticles themselves can kill bacteria, especially those in range of 3 nm. In a paper published in Nano Letters, a journal of the American Chemical Society, the Rice team demonstrated that this assumption is wrong.
According to the research team, if the chance of ionization is eliminated, these silver nanoparticles are basically harmless to microbes. The direct answer to the long-standing question is that insoluble silver nanoparticles are not able to kill cells through direct contact, whereas soluble ions that are stimulated through oxidation in the proximity of bacteria can destroy the microbes.
The research team tested both commercially available nanoparticles and custom-synthesized nanoparticles having a size range of 3-11 nm to verify the connection between toxicity and size. However, the team was not able to get reliable results. The team then tested the toxicity of nanoparticles in an anaerobic condition in order to limit the release of silver ions. What the team discovered was the toxicity of filtered particles to microbes was lesser than that of silver ions.
In order to completely eliminate the possibility of oxidation, the research team produced silver nanoparticles within the anaerobic chamber. Zongming Xiu, one of the researchers, informed that the team discovered that nanoparticles even at a concentration as high as 195 ppm were not lethal to bacteria, whereas silver ions even at a concentration of 15 ppm can completely destroy all the bacteria present. This means the toxicity of the nanoparticles is negligible as it is 7,665 folds lesser than that of the silver ions.
Moreover, the research team demonstrated that the immunity of some microbes get activated by silver ions when their dosage is not sufficient to destroy the microbes. Pedro Alvarez, one of the researchers, recommended that this anaerobic method can be used to test the toxicity of other metallic nanoparticles and to refine the antimicrobial properties of silver nanoparticles. Silver ions are the key cause of toxicity. Hence, the focus must be on controlled-release mechanisms and mass-transfer processes.
These results demonstrate that the possibility of improving silver nanoparticles’ antimicrobial application and reducing the environmental impact by controlling the release rate of silver ions, for instance, using responsive polymer coatings.