Jun 6 2014
Short, customized carbon nanotubes have the potential to deliver drugs to pancreatic cancer cells and destroy them from within, according to researchers at Rice University and the University of Texas MD Anderson Cancer Center.
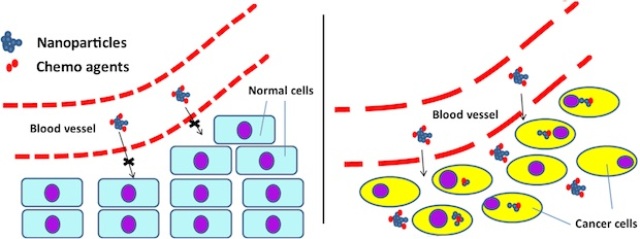 Rice researchers found that chemotherapy agents attached to nanotubes are too large to go through the pores of normal blood vessels (left), but small enough to pass through the pores of cancer-related vessels. Once through, the customized nanotubes can be taken up by cancer cells to deliver their therapeutic cargoes. Courtesy of Rei Suzuki/University of Texas MD Anderson Cancer Center)
Rice researchers found that chemotherapy agents attached to nanotubes are too large to go through the pores of normal blood vessels (left), but small enough to pass through the pores of cancer-related vessels. Once through, the customized nanotubes can be taken up by cancer cells to deliver their therapeutic cargoes. Courtesy of Rei Suzuki/University of Texas MD Anderson Cancer Center)
Pristine nanotubes produced through a new process developed at Rice can be modified to carry drugs to tumors through gaps in blood-vessel walls that larger particles cannot fit through.
The nanotubes may then target and infiltrate the cancerous cells’ nuclei, where the drugs can be released through sonication – that is, by shaking them.
The research led by Rice chemist Andrew Barron was reported in the Royal Society of Chemistry’s Journal of Materials Chemistry B. Strengthening relations with Texas Medical Center institutions is one of Rice’s Priorities for the New Century.
Most pancreatic cancer patients die within a year of diagnosis and have a five-year survival rate of 6 percent, partially because there is no method for early detection, according to the American Cancer Society. Tumors are often inoperable and pancreatic cancer cells are also difficult to reach with chemotherapy, said co-author Jason Fleming, a professor of surgical oncology at MD Anderson.
“These findings are encouraging because they offer a potential delivery solution for pancreatic cancer patients whose tumors resist standard chemotherapy,” Fleming said. “There are molecular and biological barriers to efficient delivery of chemotherapy to pancreatic cancer tumors, and these nanotubes might be able to make some of those irrelevant.”
Rice scientists made nanotubes pure enough to modify for the purpose and small enough to squeeze through the body’s defenses, Barron said. The researchers knew from previous work that nanotubes could be modified – a process called functionalization – to carry chemotherapy agents and release them at a controlled rate through sonication.
“This time, we were trying to work out how long the tubes should be and the extent of functionalization to maximize uptake by the cells,” Barron said.
Several discoveries were key, he said. First, Rice graduate student, alumnus and co-author Alvin Orbaek purified the carbon nanotubes of iron catalysts necessary to their growth by flushing them with chlorine. “Leftover iron particles damage the tubes through oxidation,” Barron said. “That makes subsequent use difficult.”
The next step was to cut the nanotubes down to size. Very long nanotubes are floppy and hard to deal with, Barron said. Enrico Andreoli, a postdoctoral research associate in Barron’s group and lead author of the paper, used a thermal process to chop them to an average length of 50 nanometers. (A human hair is about 100,000 nanometers wide.)
“Instead of ending up with a fluffy nanotube powder, we get something that looks like a hockey puck,” Barron said. “It’s not dense – it looks like a spongy puck – but you can cut it with a razor blade. You can weigh it and do accurate chemistry with it.”
Barron’s lab added polyethyleneimine (PEI) to the nanotube surfaces. In lab tests, the modified tubes were easily dispersed in liquid and able to pass through barriers into live cancer cells to infiltrate the nuclei. A small-molecule variant of PEI proved to be less toxic to cells than larger versions, Barron said.
“This research shows that the particles are small enough to get inside cells where you like them to be and that they may have an increased killing advantage – but that’s still unknown,” Fleming said.
Fleming, whose work focuses on improving drug delivery for pancreatic cancer, cautioned that more research is required. ”The next step will be to test this approach in mice that have allografts taken from human tumors,” he said. “The architecture of these tumors will more closely resemble that of human pancreatic cancer.”
Co-authors are Robert Hauge, a distinguished faculty fellow in chemistry, and Wade Adams, a senior faculty fellow in materials science and nanoengineering, both at Rice, and postdoctoral fellow Rei Suzuki and Manoop Bhutani, a professor of gastroenterology, hepatology and nutrition, both at MD Anderson. Barron is Rice’s Charles W. Duncan Jr.–Welch Professor of Chemistry and a professor of materials science and nanoengineering.
The Astellas Foundation for Research on Metabolic Disorders, the National Institutes of Health through MD Anderson’s Cancer Center Support Grant and the Robert A. Welch Foundation supported the research.