A team of researchers from the University of Illinois have created a novel material composite obtained from quantum dots.
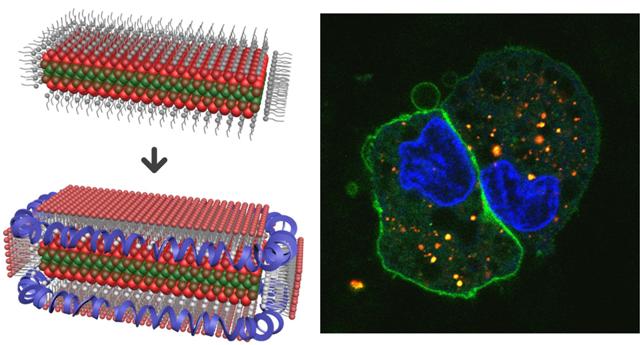 A new composite material has been made by entrapping crystalline sheets called nanoplatelets into lipoprotein nanoparticles. These lipoprotein nanoplatelets are brightly fluorescent and enter cells rapidly. Credit: Sung Jun Lim, University of Illinois
A new composite material has been made by entrapping crystalline sheets called nanoplatelets into lipoprotein nanoparticles. These lipoprotein nanoplatelets are brightly fluorescent and enter cells rapidly. Credit: Sung Jun Lim, University of Illinois
These lipoprotein nanoplatelets can be swiftly absorbed by cells without compromising their fluorescence, making them suitable for imaging cells and gaining insight into disease mechanisms.
“Quantum dots are being widely investigated due to their unique physical, optical, and electronic properties,” explained Andrew M. Smith, an assistant professor of bioengineering at Illinois. “Their most important feature is bright, stable light emission that can be tuned across a broad range of colors. This has made them useful for diverse applications as imaging agents and molecular probes in cells and tissues and as light-emitting components of LEDs and TVs.”
“These studies are the first example of flat quantum dots, called nanoplatelets, in biological systems,” said Smith, whose work is published in the Journal of the American Chemical Society.
“We have developed a unique nanoparticle that is flat, like a disc, and encapsulated within a biological particle. These are derived from quantum dots and they similarly emit light, however, they have a slew of interesting optical and structural properties because of their shape. Their light absorbing and light emitting properties are closer those of quantum wells, which are thin-layers used to make lasers. We find that these particles uniquely enter cells very rapidly and we are using them as sensors in living cells.”
“The new colloidal material is a hybrid between an inorganic quantum well and an organic nanodisc composed of phospholipids and lipoproteins,” explained Sung Jun Lim, a postdoctoral fellow in Smith’s research group and first author of the paper, 'Lipoprotein Nanoplatelets: Brightly Fluorescent, Zwitterionic Probes with Rapid Cellular Entry.'
“The phospholipids bind to the flat faces on the nanoplatelet and lipoproteins bind to curved edges to homogeneously entrap the particles in biocompatible materials. They have long-term stability in biological buffers and high salt solutions and are highly fluorescent, with brightness comparable to quantum dots when measured in a solution or at the single-molecule level in a microscope.”
Smith explains that single-molecule imaging is one ideal application of these particles, as quantum dots offer both a compact size and high light emission rate. A number of novel biological processes associated with human health and disease have been developed recently with the help of quantum dots.
“We think the new capabilities provided by nanoplatelets are valuable for imaging biological molecules and cells, but it was previously challenging to stabilize these nanocrystals in biological media because their unusual dimensions cause them to stick together, aggregate, and lose fluorescence. This new class of nanoplatelets solves these problems and they are stable under harsh biological conditions because they are encapsulated in lipoproteins.”
“We expect that this new material composite will reveal, at the single-molecule level, how flat materials interact with biological systems,” Smith added. “The unique finding of rapid cellular entry suggests that these materials may be immediately useful for cellular labeling applications to allow highly multiplexed spectral encoding of cellular identity so that we can track metastatic cancer cells in the body. Unique shapes of nanoparticles also have been found to be more efficient for delivering drugs to tumors compared with standard spherical particles, so we are exploring this as well.”
Smith’s lab and the research team of Aditi Das, an assistant professor of comparative biosciences at Illinois, have collaborated on this work. Mohammad U. Zahid, Daniel R. McDougle, Liang Ma, and Aditi Das are the other co-authors on the research paper.