Feb 24 2017
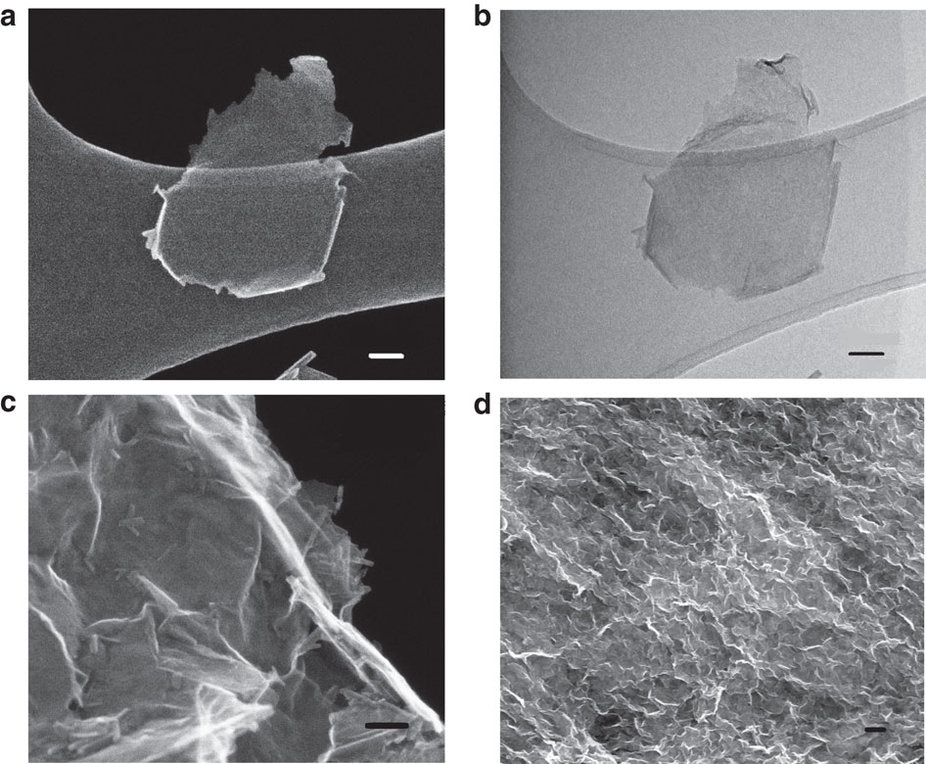 (a) SEM image and (b) bright-field TEM image of exfoliated MnO2 nanosheets, (c) high-magnification SEM image and (d) SEM image of reassembled MnO2 nanostructures treated in pH=2 solution for 24 h. Scale bar, 50 nm (a�c); 500 nm (d). CREDIT: Nature Communcations/The critical role of point defects in improving the specific capacitance of δ-MnO2 nanosheets
(a) SEM image and (b) bright-field TEM image of exfoliated MnO2 nanosheets, (c) high-magnification SEM image and (d) SEM image of reassembled MnO2 nanostructures treated in pH=2 solution for 24 h. Scale bar, 50 nm (a�c); 500 nm (d). CREDIT: Nature Communcations/The critical role of point defects in improving the specific capacitance of δ-MnO2 nanosheets
Supercapacitors are used to store energy in applications that require many rapid change/discharge cycles including personal electronics, hybrid cars, and energy harvesting systems. Supercapacitors using pseudocapacitor electrodes store energy using reversible redox reactions. Candidates for pseudocapacitor electrodes are often metal oxides due to their redox properties, combined with the high specific capacitance at low resistance.
It has long been theorized that MnO2-based materials would make ideal pseudocapacitors electrode materials for industrial supercapacitor applications due to their low cost and low toxicity, but applications of MnO2 materials have been limited by low electrical and ionic conductivities.
However, newly published research reports that a group of scientists has succeeded in producing a MnO2-based material with improved specific capacitance, charge transfer resistance, and cycling stability by introducing Mn vacancies into nanostructures built from 2D nanosheets of δ-MnO2 (birnessite).
MnO2 exists in a number of polymorphs. δ-MnO2 is a form of MnO2 with a structure that consists of sheets of edge-sharing MnO6 octahedra stacked in layers. δ-MnO2 exhibits improved capacitance and rate behavior compared with other forms of MnO2, as the space between the layers proves rapid diffusion of protons or alkali ions acting as charge carriers during the charge and discharge processes.
Despite this, and in common with the other MnO2 polymorphs, δ-MnO2 is limited by low electrical conductivity (10−5 to 10−6 S cm−1).
Attempts to improve the performance of δ-MnO2 have included the synthesis of composite materials, and nanostructuring intended to increase surface area and improve capacitance. Although it is well known that the introduction of cation vacancies can increase charge storage capacitance, thus far little research has been conducted on the effect of cation vacancies in δ-MnO2 nanosheets.
The collaboration of researchers from the US and Canada have created Mn vacancies in δ-MnO2 nanosheets using a new synthesis method that is very simple and could be used on an industrial scale. The method involves the exfoliation of δ-MnO2 nanosheets followed by re-assembly in a solution at pH 2–9.
Under carefully controlled reaction conditions, the nanosheets then form self-assembled 3D porous nanostructures which can be used as supercapacitor electrode materials.
Characterization of the resulting MnO2 nanostructures showed that the number of surface Frenkel defects (displacement of an in-plane Mn to the surface of the nanosheet) increased with decreasing pH during re-assembly of the δ-MnO2 nanosheets. The highest defect concentration (26.5%) was observed in the nanostructure comprised of δ-MnO2 nanosheets re-assembled at pH 2, compared with 19.9% in the δ-MnO2 nanosheets re-assembled at pH 4, and 18.3% in bulk HxMnO2.
Electrochemical results showed that specific capacitance of the re-assembled nanosheets correlated with the number of surface Frenkel defects, an effect that was independent of surface area.
Electrodes comprised of nanostructures of the δ-MnO2 nanosheets re-assembled at pH 2 showed the highest capacitance (300 F g-1), the highest stability (83% of its initial capacitance remained after 1,000 charge-discharge cycles at 5 A g-1) and the lowest charge transfer resistance (~3 Ω).
The combined results suggest that the increased number of surface Frenkel defects in the nanosheets re-assembled at pH 2 resulted in increased Na+ ion intercalation by providing low energy intercalation sites, possibly due to local distortions in the nanosheets around the vacancies.
This is the first time that researchers have correlated the number of defects in MnO2 with capacitance, and therefore represents a step towards the industrial application of MnO2-based supercapacitors.
However, while this production method for δ-MnO2 nanosheets with improved electrochemical properties is simple and could be applied on an industrial scale, MnO2-based materials for supercapacitors still face challenges such as reductions in capacity upon cycling, and low capacitance compared with other metal oxides such as RuO2.
Source:
Gao P., Metz P., Hey T., Gong Y., Liu D., Edwards D.D., Howe J.Y., Huang R., Misture S.T., The critical role of point defects in improving the specific capacitance of δ-MnO2 nanosheets, Nature Communications 8, 2017, 14559