Sep 7 2020
In electrocatalysis processes, single-atom catalysts (SACs) exhibit high potential as they tend to utilize atomic-scale active species to the maximum possible extent.
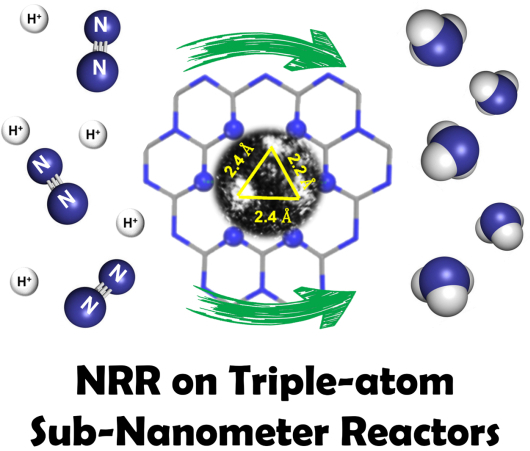 TEM image of a triple-atom active center and the schematic illustration of a sub-nanometer reactor hosting it for catalyzing NRR. Image Credit: LIANG Ji.
TEM image of a triple-atom active center and the schematic illustration of a sub-nanometer reactor hosting it for catalyzing NRR. Image Credit: LIANG Ji.
However, so far, it has been highly challenging to manipulate these atomic-scale active sites to achieve particular reactions. The reason behind this is that they tend to exhibit isolation features.
At the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, Prof. LIU Jian and his colleagues put forward a novel nano-confinement method that involves introducing several Fe and Cu single atoms into the extremely narrow yet typical surface cavities of graphitic carbon nitride to create what are called “sub-nanometer reactors.”
The research was reported in the Advanced Materials journal on September 2nd, 2020.
These Fe and Cu atoms, highly confined in the sub-nanometer reactors, not only provide stronger interaction with the reactants but also, more importantly, lead to significant synergetic effect due to their unique microenvironments in this extremely narrow space, which is highly favorable for catalysis, especially the tandem processes such as the nitrogen reduction reaction.
LIANG Ji, Study Co-Author and Professor, Tianjin University
Prof. LIU noted that “This is the first time that we successfully and conceptually push the nanoreactors towards a much smaller dimension to form sub-nanometer reactors, which brings distinctively different properties from the conventional nanoreactors.”
First principle simulation reveals that this synergistic effect originates from the unique Fe-Cu coordination, which effectively modifies N2 absorption, improves electron transfer, and offers extra redox couples for nitrogen reduction reaction.
SUN Chenghua, Study Co-Author and Professor, Swinburne University of Technology
As part of the study, it was discovered by the researchers that this crucial synergy brought about by the multiple confined atoms resulted in considerable improvement in the performance for the nitrogen reduction reaction (NRR), which is a model electrocatalytic process.
The researchers achieved enhancements in terms of high ammonia yield and efficiency, which are considerably higher when compared to the mono-metal equivalents.
Apart from offering a new approach through which the active centers of catalysts can be manipulated at the subnanometer scale, this concept of developing sub-nanometer reactors also offers insights into ways to design novel catalysts with an accurate spatial location at the sub-nanometer scale, which also helps perform a wide range of catalytic reactions.
Journal Reference:
Wang, X., et al. (2020) Confined Fe-Cu Clusters as Sub‐Nanometer Reactors for Efficiently Regulating the Electrochemical Nitrogen Reduction Reaction. Advanced Materials. doi.org/10.1002/adma.202004382.