Shape is turning out to be a particularly important feature of some commercially important nanoparticles-but in subtle ways. New studies* by scientists at the National Institute for Standards and Technology (NIST) show that changing the shape of cobalt nanoparticles from spherical to cubic can fundamentally change their behavior.
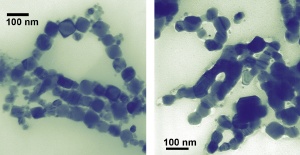 These cubes of cobalt (left/top), measuring about 50 nanometers wide, are showing scientists that, on the nanoscale, a change in shape is a change in property. Unlike smaller spherical cobalt nanoparticles, nanocubes melt and fuse (right/bottom) when illuminated by a transmission electron microscope and possess different magnetic characteristics than the nanospheres as well. Credit: NIST
These cubes of cobalt (left/top), measuring about 50 nanometers wide, are showing scientists that, on the nanoscale, a change in shape is a change in property. Unlike smaller spherical cobalt nanoparticles, nanocubes melt and fuse (right/bottom) when illuminated by a transmission electron microscope and possess different magnetic characteristics than the nanospheres as well. Credit: NIST
Building on a previous paper** that examined the properties of cobalt formed into spheres just a few nanometers in diameter, the new work explores what happens when the cobalt is synthesized instead as nanocubes. Nanoparticles of cobalt possess large magnetic moments—a measure of magnetic strength—and unique catalytic properties, and have potential applications in information storage, energy and medicine.
One striking difference is the behavior of the two different particle types when external magnetic fields are applied and then removed. In the absence of a magnetic field, both the spherical and cubic nanoparticles spontaneously form chains—lining up as a string of microscopic magnets. Then, when placed in an external magnetic field, the individual chains bundle together in parallel lines to form thick columns aligned with the field. These induced columns, says NIST physicist Angela Hight Walker, imply that the external magnetic fields have a strong impact on the magnetic behavior of both nanoparticle shapes.
But their group interactions are somewhat different. As the strength of the external field is gradually reduced to zero, the magnetization of the spherical nanoparticles in the columns also decreases gradually. On the other hand, the magnetization of the cubic particles in the columns decreases in a much slower fashion until the particles rearrange their magnetic moments from linear chains into small circular groups, resulting in a sudden drop in their magnetization.
The team also showed that the cubes can be altered merely by observing with one of nanotechnology’s microscopes of choice. After a few minutes’ exposure to the illuminating beam of a transmission electron microscope, the nanocubes melt together, forming “nanowires” that are no longer separable as individual nanoparticles. The effect, not observed with the spheres, is surprising because the cubes average 50 nm across, much larger than the spheres’ 10 nm diameters. “You might expect the smaller objects to have a lower melting point,” Hight Walker says. “However, the sharp edges and corners in the nanocubes could be the locations to initiate melting.”
While Walker says that the melting effect could be a potential method for fabricating nanostructures, it also demands further attention. “This newfound effect demonstrates the need to characterize the physico-chemical properties of nanoparticles extremely well in order to pursue their applications in biology and medicine,” she says.
* G. Cheng, R.D. Shull and A.R. Hight Walker. Dipolar chains formed by chemically synthesized cobalt nanocubes. Journal of Magnetism and Magnetic Materials, May 11, 2009, Vol. 321, issue 10, pp. 1351—1355.
** G. Cheng, D. Romero, G.T. Fraser and A.R. Hight Walker. Magnetic-field-induced assemblies of cobalt nanoparticles. Langmuir, December 2005. See Oct. 20, 2007, Tech Beat article, “Magnetic Nanoparticles Assembled into Long Chains”.