Jun 26 2009
An international team, led by Shingo Nagano from the RIKEN SPring-8 Center in Harima and Hiroyasu Onaka from Toyama Prefectural University, has uncovered the vital role of water in the generation of the antitumor drug staurosporine*.
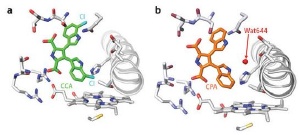 The crystal structures of the binding pockets in StaP containing (a) CCA in which water (Wat644) is absent, and (b) CPA.
The crystal structures of the binding pockets in StaP containing (a) CCA in which water (Wat644) is absent, and (b) CPA.
The researchers mainly focus on the enzyme P450 StaP, which belongs to the cytochrome P450 enzyme family. These enzymes are involved in metabolic and biosynthetic reactions, including the activation and degradation of drugs in humans, and the synthesis of medically relevant natural products.
P450 StaP’s active site consists of a sulfur-bound iron atom enclosed in a large hydrocarbon ring called heme. It catalyzes the oxidation of a five-ring compound called chromopyrrolic acid (CPA) and facilitates the formation of an intramolecular carbon–carbon bond to generate a six-ring staurosporine precursor. This carbon–carbon bond formation is unusual for P450 enzymes, which typically insert an oxygen atom into bonds. The researchers demonstrated that water molecules mediate this carbon–carbon coupling.
Nagano and co-workers had previously revealed that strong interactions held CPA tightly in a binding pocket, modulating proton and electron transfer reactions between substrate and enzyme. However, they observed that those interactions kept the substrate away from the heme oxygen, impeding any direct contact, and thus proton transfer, between the two species.
In their latest work, they mutated the enzyme by replacing a residue positioned between the two water molecules with hydrocarbons, which significantly decreased its activity. They also substituted CPA with a chlorine-containing compound (CCA) and discovered that the chlorine atom prevented water molecules from approaching the heme. Further, they observed decreased activity in presence of CCA, highlighting the importance of water in the mechanism.
“CCA is very poor substrate but we had no idea why this happens,” says Nagano. Since his collaborator proposed that this water molecule was very likely to be a key player in this enzyme catalysis, they ran a detailed computational investigation. They found that two water molecules in the enzyme active site acted as a proton relay between CPA and the heme.
“Similar water-assisted proton transfer between heme and substrate is also found in horseradish peroxidase (HRP), another heme enzyme,” explains Nagano. “The natural substrate-bound HRP has a water molecule close to the substrate and heme as we have observed in CPA-bound P450 StaP.” The researchers’ ultimate goal is to transpose this carbon–carbon coupling to other P450 enzymes and generate new staurosporine-like therapeutic agents.
*Wang, Y., Chen, H., Makino, M., Shiro, Y., Nagano, S., Asamizu, S., Onaka, H. & Shaik, S. Theoretical and experimental studies of the conversion of chromopyrrolic acid to an antitumor derivative by cytochrome P450 StaP: the catalytic role of water molecules. Journal of the American Chemical Society 131, 6748–6762 (2009).