Jul 31 2009
A group of nanoengineers, biologists and physicists have used innovative approaches to deduce the internal structure of chromatin, a key player in DNA regulation, to reconcile a longstanding controversy in this field. This new finding could unlock the mystery behind the origin of many diseases such as cancer.
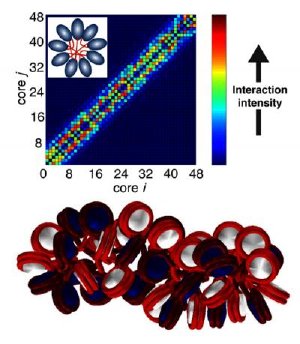 UCSD nanoengineering professor Gaurav Arya has developed new computational models of chromatin -- a key player in DNA regulation -- that suggests that its structure is heteromorphic (bottom graphic) from anaylsis of the interaction pattern between nucleosomes in the fiber (top graphic). Credit: UC San Diego
UCSD nanoengineering professor Gaurav Arya has developed new computational models of chromatin -- a key player in DNA regulation -- that suggests that its structure is heteromorphic (bottom graphic) from anaylsis of the interaction pattern between nucleosomes in the fiber (top graphic). Credit: UC San Diego
The details of this breakthrough discovery are highlighted in a paper in the Proceedings of the National Academy of Sciences (PNAS) called "Evidence for heteromorphic chromatin fibers from analysis of nucleosome interactions." The authors include Gaurav Arya, a nanoengineering professor at the UC San Diego Jacobs School of Engineering, as well as researchers from Penn State University, University of Massachusetts, and New York University.
The internal structure of chromatin is not known—a mystery that has baffled scientists for more than three decades. Chromatin is a complex combination of DNA and proteins that makes up chromosomes. The function of chromatin is to package DNA into a smaller volume to fit in the cell. Chromatin also plays an important role in the regulation of genetic processes like DNA replication, transcription, recombination, and repair because all these processes depend critically on the accessibility of the DNA, which is directly controlled by chromatin. A loosely folded chromatin fiber allows easy access to DNA sequences while a tightly folded fiber prevents or inhibits such access.
This newfound discovery by Arya and his colleagues could help scientists better understand how chromatin folds and unfolds to regulate gene activities, as well as understand the origin of genetic diseases like cancer. For example, he said, lots of diseases including cancer are directly linked to abnormal regulation of chromatin.
The structure of chromatin has been a subject of many controversies during the last 30 years, with two different models being the focus of debate. The new findings by Arya and his collaborators suggest that the structure of chromatin is actually a combination of both of these models.
Some researchers suggest that its structure is like a solenoid, where the chain of nucleosomes follows a helical path along the chromatin axis such that adjacent nucleosomes along the chain lie side by side on the chromatin fiber also and thus the linker DNA connecting the two needs to maintain a highly bent conformation. Other researchers suggest that chromatin has a zigzag structure where the nucleosome chain follows a zigzag path along the chromatin fiber such that adjacent nucleosomes on the chain actually lie on opposite sides of the fiber such that the linker DNA is straight. (The nucleosome is the basic unit of chromatin that consists of two turns of DNA wrapped around a protein spool).
Arya and his team have developed a very sophisticated model of chromatin that is computationally accessible yet detailed enough to provide insights into its structure and dynamics. By simulating this model under physiological conditions using fast computer algorithms, Arya has provided the first evidence that chromatin does not exist uniquely in solenoid or zigzag forms but rather exhibits a "heteromorphic" structure where both types of structures are in balance with each other. Arya's collaborators have developed an innovative experimental approach called Electron microscopy assisted nucleosome capture technique to corroborate Arya's findings.
"What we did was extract the most important features of DNA and the associated proteins in the model, using techniques called coarse-graining," Arya explained. "You cannot simulate something as large as a chromatin fiber containing tens of nucleosomes at molecular detail for periods of time longer than a few nanoseconds. That will not tell you much. You need to reduce the complexity of the system without sacrificing important details, which is exactly what we have done. We're trying to reduce the total number of variables in the system by eliminating the variables that are not important at the length and time scales of interest. "This research emphasizes how engineering tools can provide new insights into biology," he added.
"When we talk about cancer and its link to abnormal gene regulation, we have to ask the question why are these genes misfiring? In the end, it boils down to the protein machinery that makes up and regulates chromatin," he explained. "You could have mutations in the oncogenic or tumor suppressor genes that lead to cancer or you could have abnormalities in the proteins that regulate chromatin around these genes leading to cancer."
The next step for Arya and his team is to study the mechanisms by which chromatin is regulated, and how those lead to genes being switched "on" and "off," genes being repaired in response to mutations, and genes being replicated during cell division. Arya believes that a molecular-level understanding of such mechanisms could lead to the development of better drugs that will directly target the chromatin around abnormally regulated genes to correct their activity. "In fact, there are already drugs being developed called HDIs (histone deacetylase inhibitors) that can modulate structure of chromatin around oncogenic genes so as to inhibit cancer progression, though their exact mechanisms are not fully known," Arya said.
"The scientific community is quite fascinated by DNA and they tend to forget that DNA is rarely present in its naked double-stranded helix form in higher organisms like humans, but that it is always associated with proteins in this complex aggregate we call chromatin," he added. "Undoubtedly, DNA codes for nearly all the proteins and RNAs manufactured inside our cells but how, when, and which genes are turned on and off is almost completely determined by the structure, dynamics, and chemical properties of chromatin. …We now know how chromatin compacts and what its internal structure looks like. "But we are far from solving the mystery of the mechanisms by which chromatin exercises dynamic control over DNA."
PNAS paper, "Evidence for heteromorphic chromatin fibers from analysis of nucleosome interactions," by UCSD nanoengineering professor Gaurav Arya, Tamar Schlick of New York University, Sergei Grigoryev and Sarah Corell from Penn State University, and Chris Woodcock from the University of Massachusetts.