A research team at the National Institute of Standards and Technology (NIST) studying sugar-coated nanoparticles for use as a possible cancer therapy has uncovered a delicate balancing act that makes the particles more effective than conventional thinking says they should be. Just like individuals in a crowd respecting other people's personal space, the particles work because they get close together, but not too close.
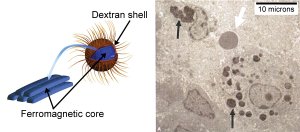 An iron-centered nanoparticle (left) analyzed at NIST’s Center for Neutron Research has a coating of the sugar dextran, whose tendrils prevent groups of the particles from clumping. When tumor cells ingest them (right), the particles still congregate closely enough to share heat when stimulated by a magnetic field, killing the cells. White arrow indicates a red blood cell. Credit: (l.) J. Aarons; (r.) A. Guistini, R. Strawbridge and P. Hoopes, Dartmouth College
An iron-centered nanoparticle (left) analyzed at NIST’s Center for Neutron Research has a coating of the sugar dextran, whose tendrils prevent groups of the particles from clumping. When tumor cells ingest them (right), the particles still congregate closely enough to share heat when stimulated by a magnetic field, killing the cells. White arrow indicates a red blood cell. Credit: (l.) J. Aarons; (r.) A. Guistini, R. Strawbridge and P. Hoopes, Dartmouth College
In cooperation with colleagues at The Johns Hopkins University, Dartmouth College, the University of Manitoba and two biopharmaceutical companies, the NIST team has demonstrated* that the particles—essentially sugar-coated bits of iron oxide, about 100 nanometers wide—are potent cancer killers because they interact with one another in ways that smaller nanoparticles do not. The interactions, thought by many bioengineers to be undesirable, actually help the larger particles heat up better when subjected to an alternating magnetic field. Because this heat destroys cancer cells, the team's findings may help engineers design better particles and treatment methods.
Nanoparticles hold the promise of battling cancer without the damaging side effects of chemotherapy or radiation treatment. Minuscule balls of iron oxide can be coated with sugar molecules making them particularly attractive to resource-hungry cancer cells. Once the particles are injected, cancer cells would then ingest them, and doctors would then be able to apply an alternating magnetic field that causes the iron oxide centers to heat, killing the cancer but leaving surrounding tissue unharmed.
Two biotech companies, Micromod Partikeltechnologie and Aduro BioTech, created particles that showed great potential in treating cancers in mice, and they asked NIST to help understand why it worked so well. “But they sent us particles that were much larger than what the conventional wisdom says they should be,” says NIST materials scientist Cindi Dennis. “Larger particles are more strongly magnetic and tend to clump together, which makes them large enough to attract the body's defense systems before they can reach a tumor. The companies' nanoparticles, however, did not have this problem.”
Neutron scattering probes at the NIST Center for Neutron Research revealed that the particles' larger iron oxide cores attract one another, but that the sugar coating has fibers extending out, making it resemble a dandelion—and these fibers push against one another when two particles get too close together, making them spring apart and maintain an antibody-defying distance rather than clumping. Moreover, when the particles do get close, the iron oxide centers all rotate together under the influence of a magnetic field, both generating more heat and depositing this heat locally. All these factors helped the nanoparticles destroy breast tumors in three out of four mice after one treatment with no regrowth.
“The push-pull is part of a tug of war that fixes the distance between nanoparticles,” Dennis says. “This suggests we can stabilize interacting particles in ways that potentially pay off in the clinic.”
The research was funded by the U.S. Army Medical Research and Materiel Command and used facilities supported by the National Science Foundation.
* C.L. Dennis, A.J. Jackson, J.A. Borchers, P.J. Hoopes, R. Strawbridge, A.R. Foreman, J. van Lierop, C. Gruttner and R. Ivkov. Nearly complete regression of tumors via collective behavior of magnetic nanoparticles in hyperthermia. Nanotechnology, 20 (2009) 395103. [doi:10.1088/0957-4484/20/39/395103]