Oct 7 2009
Wires with atomic dimensions are potential structural elements for future nanoscopic electronic components. Such fine wires have completely new electronic properties. However, apart from the non-trivial production of metallic nanowires, their high chemical reactivity is a critical problem; they are easily oxidized in air and are not stable. Japanese researchers working with R. Kitaura and H. Shinohara have now developed a new method that is simple and delivers stable nanowires: They deposit metal atoms inside of carbon nanotubes. As the scientists report in the journal Angewandte Chemie, this forms metal wires of individual atoms lined up side-by-side that are so well protected by their sheath that they have long-term stability.
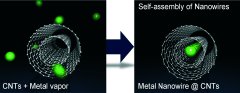
The method of production simply involves heating carbon nanotubes and a metal powder together in a vacuum. It works for all metals that enter into a gaseous phase at relatively low temperatures, such as europium, samarium, ytterbium, and strontium. The metal atoms almost completely fill the cavity inside the carbon nanotubes. Using europium metal and carbon nanotubes with an inner diameter of about 0.76 nm, the researchers were able to obtain wires made of a single chain of individual atoms. This first true one-dimensional nanowires was also stable after one month of exposure to air.
By using carbon nanotubes with different inner diameters, ultrafine wires with various diameters could be produced, which were for example formed of two or four atomic chains. In comparison to macroscopic europium crystals, the atomic wires demonstrate significantly different electronic and magnetic properties.
The nanowires are an ideal model for the study of one-dimensional phenomena. The researchers now aim to test the properties of the wires with respect to their suitability for use as “wiring” for nanoelectronic components.